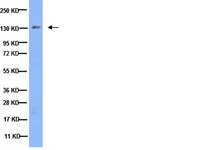
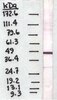
Integrin α3β1 regulates kidney collecting duct development via TRAF6-dependent K63-linked polyubiquitination of Akt.
Yazlovitskaya, EM; Tseng, HY; Viquez, O; Tu, T; Mernaugh, G; McKee, KK; Riggins, K; Quaranta, V; Pathak, A; Carter, BD; Yurchenco, P; Sonnenberg, A; Böttcher, RT; Pozzi, A; Zent, R
Molecular biology of the cell
26
1857-74
2015
Afficher le résumé
The collecting system of the kidney develops from the ureteric bud (UB), which undergoes branching morphogenesis, a process regulated by multiple factors, including integrin-extracellular matrix interactions. The laminin (LM)-binding integrin α3β1 is crucial for this developmental program; however, the LM types and LM/integrin α3β1-dependent signaling pathways are poorly defined. We show that α3 chain-containing LMs promote normal UB branching morphogenesis and that LM-332 is a better substrate than LM-511 for stimulating integrin α3β1-dependent collecting duct cell functions. We demonstrate that integrin α3β1-mediated cell adhesion to LM-332 modulates Akt activation in the developing collecting system and that Akt activation is PI3K independent but requires decreased PTEN activity and K63-linked polyubiquitination. We identified the ubiquitin-modifying enzyme TRAF6 as an interactor with the integrin β1 subunit and regulator of integrin α3β1-dependent Akt activation. Finally, we established that the developmental defects of TRAF6- and integrin α3-null mouse kidneys are similar. Thus K63-linked polyubiquitination plays a previously unrecognized role in integrin α3β1-dependent cell signaling required for UB development and may represent a novel mechanism whereby integrins regulate signaling pathways. | | | 25808491
 |
Mitochondrial uncoupling links lipid catabolism to Akt inhibition and resistance to tumorigenesis.
Nowinski, SM; Solmonson, A; Rundhaug, JE; Rho, O; Cho, J; Lago, CU; Riley, CL; Lee, S; Kohno, S; Dao, CK; Nikawa, T; Bratton, SB; Wright, CW; Fischer, SM; DiGiovanni, J; Mills, EM
Nature communications
6
8137
2015
Afficher le résumé
To support growth, tumour cells reprogramme their metabolism to simultaneously upregulate macromolecular biosynthesis while maintaining energy production. Uncoupling proteins (UCPs) oppose this phenotype by inducing futile mitochondrial respiration that is uncoupled from ATP synthesis, resulting in nutrient wasting. Here using a UCP3 transgene targeted to the basal epidermis, we show that forced mitochondrial uncoupling inhibits skin carcinogenesis by blocking Akt activation. Similarly, Akt activation is markedly inhibited in UCP3 overexpressing primary human keratinocytes. Mechanistic studies reveal that uncoupling increases fatty acid oxidation and membrane phospholipid catabolism, and impairs recruitment of Akt to the plasma membrane. Overexpression of Akt overcomes metabolic regulation by UCP3, rescuing carcinogenesis. These findings demonstrate that mitochondrial uncoupling is an effective strategy to limit proliferation and tumorigenesis through inhibition of Akt, and illuminate a novel mechanism of crosstalk between mitochondrial metabolism and growth signalling. | Western Blotting | | 26310111
 |
MicroRNA-4723 inhibits prostate cancer growth through inactivation of the Abelson family of nonreceptor protein tyrosine kinases.
Arora, S; Saini, S; Fukuhara, S; Majid, S; Shahryari, V; Yamamura, S; Chiyomaru, T; Deng, G; Tanaka, Y; Dahiya, R
PloS one
8
e78023
2013
Afficher le résumé
The Abelson (c-Abl) proto-oncogene encodes a highly conserved nonreceptor protein tyrosine kinase that plays a role in cell proliferation, differentiation, apoptosis and cell adhesion. c-Abl represents a specific anti-cancer target in prostate cancer as aberrant activity of this kinase has been implicated in the stimulation of prostate cancer growth and progression. However, the mechanism of regulation of c-Abl is not known. Here we report that Abl kinases are regulated by a novel microRNA, miR-4723, in prostate cancer. Expression profiling of miR-4723 expression in a cohort of prostate cancer clinical specimens showed that miR-4723 expression is widely attenuated in prostate cancer. Low miR-4723 expression was significantly correlated with poor survival outcome and our analyses suggest that miR-4723 has significant potential as a disease biomarker for diagnosis and prognosis in prostate cancer. To evaluate the functional significance of decreased miR-4723 expression in prostate cancer, miR-4723 was overexpressed in prostate cancer cell lines followed by functional assays. miR-4723 overexpression led to significant decreases in cell growth, clonability, invasion and migration. Importantly, miR-4723 expression led to dramatic induction of apoptosis in prostate cancer cell lines suggesting that miR-4723 is a pro-apoptotic miRNA regulating prostate carcinogenesis. Analysis of putative miR-4723 targets showed that miR-4723 targets integrin alpha 3 and Methyl CpG binding protein in addition to Abl1 and Abl2 kinases. Further, we found that the expression of Abl kinase is inversely correlated with miR-4723 expression in prostate cancer clinical specimens. Also, Abl1 knockdown partially phenocopies miR-4723 reexpression in prostate cancer cells suggesting that Abl is a functionally relevant target of miR-4723 in prostate cancer. In conclusion, we have identified a novel microRNA that mediates regulation of Abl kinases in prostate cancer. This study suggests that miR-4723 may be an attractive target for therapeutic intervention in prostate cancer. | Western Blotting | | 24223753
 |
SEL1L regulates adhesion, proliferation and secretion of insulin by affecting integrin signaling.
Diaferia, GR; Cirulli, V; Biunno, I
PloS one
8
e79458
2013
Afficher le résumé
SEL1L, a component of the endoplasmic reticulum associated degradation (ERAD) pathway, has been reported to regulate the (i) differentiation of the pancreatic endocrine and exocrine tissue during the second transition of mouse embryonic development, (ii) neural stem cell self-renewal and lineage commitment and (iii) cell cycle progression through regulation of genes related to cell-matrix interaction. Here we show that in the pancreas the expression of SEL1L is developmentally regulated, such that it is readily detected in developing islet cells and in nascent acinar clusters adjacent to basement membranes, and becomes progressively restricted to the islets of Langherans in post-natal life. This peculiar expression pattern and the presence of two inverse RGD motifs in the fibronectin type II domain of SEL1L protein indicate a possible interaction with cell adhesion molecules to regulate islets architecture. Co-immunoprecipitation studies revealed SEL1L and ß1-integrin interaction and, down-modulation of SEL1L in pancreatic ß-cells, negatively influences both cell adhesion on selected matrix components and cell proliferation likely due to altered ERK signaling. Furthermore, the absence of SEL1L protein strongly inhibits glucose-stimulated insulin secretion in isolated mouse pancreatic islets unveiling an important role of SEL1L in insulin trafficking. This phenotype can be rescued by the ectopic expression of the ß1-integrin subunit confirming the close interaction of these two proteins in regulating the cross-talk between extracellular matrix and insulin signalling to create a favourable micro-environment for ß-cell development and function. | | | 24324549
 |
Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system.
Chon, BH; Lee, EJ; Jing, L; Setton, LA; Chen, J
Stem cell research & therapy
4
120
2013
Afficher le résumé
Cell supplementation to the herniated or degenerated intervertebral disc (IVD) is a potential strategy to promote tissue regeneration and slow disc pathology. Human umbilical cord mesenchymal stromal cells (HUCMSCs) - originating from the Wharton's jelly - remain an attractive candidate for such endeavors with their ability to differentiate into multiple lineages. Previously, mesenchymal stem cells (MSCs) have been studied as a potential source for disc tissue regeneration. However, no studies have demonstrated that MSCs can regenerate matrix with unique characteristics matching that of immature nucleus pulposus (NP) tissues of the IVD. In our prior work, immature NP cells were found to express specific laminin isoforms and laminin-binding receptors that may serve as phenotypic markers for evaluating MSC differentiation to NP-like cells. The goal of this study is to evaluate these markers and matrix synthesis for HUCMSCs cultured in a laminin-rich pseudo-three-dimensional culture system.HUCMSCs were seeded on top of Transwell inserts pre-coated with Matrigel™, which contained mainly laminin-111. Cells were cultured under hypoxia environment with three differentiation conditions: NP differentiation media (containing 2.5% Matrigel™ solution to provide for a pseudo-three-dimensional laminin culture system) with no serum, or the same media supplemented with either insulin-like growth factor-1 (IGF-1) or transforming growth factor-β1 (TGF-β1). Cell clustering behavior, matrix production and the expression of NP-specific laminin and laminin-receptors were evaluated at days 1, 7, 13 and 21 of culture.Data show that a pseudo-three-dimensional culture condition (laminin-1 rich) promoted HUCMSC differentiation under no serum conditions. Starting at day 1, HUCMSCs demonstrated a cell clustering morphology similar to that of immature NP cells in situ and that observed for primary immature NP cells within the similar laminin-rich culture system (prior study). Differentiated HUCMSCs under all conditions were found to contain glycosaminoglycan, expressed extracellular matrix proteins of collagen II and laminin α5, and laminin receptors (integrin α3 and β4 subunits). However, neither growth factor treatment generated distinct differences in NP-like phenotype for HUCMSC as compared with no-serum conditions.HUCMSCs have the potential to differentiate into cells sharing features with immature NP cells in a laminin-rich culture environment and may be useful for IVD cellular therapy. | | | 24405888
 |
Viral infection of the pregnant cervix predisposes to ascending bacterial infection.
Racicot, K; Cardenas, I; Wünsche, V; Aldo, P; Guller, S; Means, RE; Romero, R; Mor, G
Journal of immunology (Baltimore, Md. : 1950)
191
934-41
2013
Afficher le résumé
Preterm birth is the major cause of neonatal mortality and morbidity, and bacterial infections that ascend from the lower female reproductive tract are the most common route of uterine infection leading to preterm birth. The uterus and growing fetus are protected from ascending infection by the cervix, which controls and limits microbial access by the production of mucus, cytokines, and antimicrobial peptides. If this barrier is compromised, bacteria may enter the uterine cavity, leading to preterm birth. Using a mouse model, we demonstrate, to our knowledge for the first time, that viral infection of the cervix during pregnancy reduces the capacity of the female reproductive tract to prevent bacterial infection of the uterus. This is due to differences in susceptibility of the cervix to infection by virus during pregnancy and the associated changes in TLR and antimicrobial peptide expression and function. We suggest that preterm labor is a polymicrobial disease, which requires a multifactorial approach for its prevention and treatment. | Western Blotting | | 23752614
 |
Integrin upregulation and localization to focal adhesion sites in pregnant human myometrium.
Burkin, HR; Rice, M; Sarathy, A; Thompson, S; Singer, CA; Buxton, IL
Reproductive sciences (Thousand Oaks, Calif.)
20
804-12
2013
Afficher le résumé
Focal adhesions are integrin-rich microdomains that structurally link the cytoskeleton to the extracellular matrix and transmit mechanical signals. In the pregnant uterus, increases in integrin expression and activation are thought to be critical for the formation of the mechanical syncytium required for labor. The aim of this study was to determine which integrins are upregulated and localized to focal adhesions in pregnant human myometrium. We used quantitative polymerase chain reaction, Western blotting, and confocal microscopy to determine the expression levels and colocalization with focal adhesion proteins. We observed increases in several integrin transcripts in pregnant myometrium. At the protein level, integrins such as α5-integrin (ITGA5), ITGA7, ITGAV, and ITGB3 were significantly increased during pregnancy. The integrins ITGA3, ITGA5, ITGA7, and ITGB1 colocalized with focal adhesion proteins in term human myometrium. These data suggest that integrins α3β1, α5β1, and α7β1 are the most likely candidates to transmit mechanical signals from the extracellular matrix through focal adhesions in pregnant human myometrium. | | | 23298868
 |
Adhesion proteins--an impact on skeletal myoblast differentiation.
Przewoźniak, M; Czaplicka, I; Czerwińska, AM; Markowska-Zagrajek, A; Moraczewski, J; Stremińska, W; Jańczyk-Ilach, K; Ciemerych, MA; Brzoska, E
PloS one
8
e61760
2013
Afficher le résumé
Formation of mammalian skeletal muscle myofibers, that takes place during embryogenesis, muscle growth or regeneration, requires precise regulation of myoblast adhesion and fusion. There are few evidences showing that adhesion proteins play important role in both processes. To follow the function of these molecules in myoblast differentiation we analysed integrin alpha3, integrin beta1, ADAM12, CD9, CD81, M-cadherin, and VCAM-1 during muscle regeneration. We showed that increase in the expression of these proteins accompanies myoblast fusion and myotube formation in vivo. We also showed that during myoblast fusion in vitro integrin alpha3 associates with integrin beta1 and ADAM12, and also CD9 and CD81, but not with M-cadherin or VCAM-1. Moreover, we documented that experimental modification in the expression of integrin alpha3 lead to the modification of myoblast fusion in vitro. Underexpression of integrin alpha3 decreased myoblasts' ability to fuse. This phenomenon was not related to the modifications in the expression of other adhesion proteins, i.e. integrin beta1, CD9, CD81, ADAM12, M-cadherin, or VCAM-1. Apparently, aberrant expression only of one partner of multiprotein adhesion complexes necessary for myoblast fusion, in this case integrin alpha3, prevents its proper function. Summarizing, we demonstrated the importance of analysed adhesion proteins in myoblast fusion both in vivo and in vitro. | Immunofluorescence | | 23671573
 |
Alteration of the actin cytoskeleton and localisation of the α6β1 and α3 integrins during regeneration of the rat submandibular gland.
Osamu Shimizu,Hiroshi Shiratsuchi,Koichiro Ueda,Shunichi Oka,Yoshiyuki Yonehara
Archives of oral biology
57
2011
Afficher le résumé
Actin filaments, which are regulated by signal transduction via integrins, play important roles in the regulation of cell differentiation and polarity. The aim of this study was to assess alterations in the cytoskeleton and the localisation of integrins during regeneration of the rat submandibular gland. | | | 22410146
 |
Regulation of integrin endocytic recycling and chemotactic cell migration by syntaxin 6 and VAMP3 interaction.
Riggs, KA; Hasan, N; Humphrey, D; Raleigh, C; Nevitt, C; Corbin, D; Hu, C
Journal of cell science
125
3827-39
2011
Afficher le résumé
Integrins are the primary receptors of cells adhering to the extracellular matrix, and play key roles in various cellular processes including migration, proliferation and survival. The expression and distribution of integrins at the cell surface is controlled by endocytosis and recycling. The present study examines the function of syntaxin 6 (STX6), a t-SNARE located in the trans-Golgi network, in integrin trafficking. STX6 is overexpressed in many types of human cancer. We show that depletion of STX6 inhibits chemotactic cell migration and the delivery of the laminin receptor α3β1 integrin to the cell surface, whereas STX6 overexpression stimulates chemotactic cell migration, integrin delivery, and integrin-initiated activation of focal adhesion kinase. These data indicate that STX6 plays a rate-limiting role in cell migration and integrin trafficking. In STX6-depleted cells, α3β1 integrin is accumulated in recycling endosomes that contain the v-SNARE VAMP3. Importantly, we show that STX6 and VAMP3 form a v-/t-SNARE complex, VAMP3 is required in α3β1 integrin delivery to the cell surface, and endocytosed α3β1 integrin traffics to both VAMP3 and STX6 compartments. Collectively, our data suggest a new integrin trafficking pathway in which endocytosed integrins are transported from VAMP3-containing recycling endosomes to STX6-containing trans-Golgi network before being recycled to the plasma membrane. | | | 22573826
 |