565790 Sigma-Aldrichγ-Secretase Inhibitor XXI, Compound E - CAS 209986-17-4 - Calbiochem
This g-secretase inhibitor, CAS 209986-17-4, is a cell-permeable, potent, selective, non-transition-state analog inhibitor of γ-secretase and Notch processing. Lowers Aβ levels in APP transgenic mice
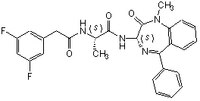
More>> This g-secretase inhibitor, CAS 209986-17-4, is a cell-permeable, potent, selective, non-transition-state analog inhibitor of γ-secretase and Notch processing. Lowers Aβ levels in APP transgenic mice Less<<Synonymes: (S,S)- 2-[2-(3,5-Difluorophenyl)-acetylamino]-N-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-propionamide, Compound E
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 209986-17-4 | C₂₇H₂₄F₂N₄O₃ |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 565790-1MG |
|
Flacon en verre | 1 mg |
|
— | |
| 565790-500UG |
|
Ampoule plast. | 500 μg |
|
— |
| Product Information | |
|---|---|
| CAS number | 209986-17-4 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₂₇H₂₄F₂N₄O₃ |
| Chemical formula | C₂₇H₂₄F₂N₄O₃ |
| Reversible | N |
| Structure formula Image | |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 565790-1MG | 04055977191851 |
| 565790-500UG | 04055977191868 |
Documentation
γ-Secretase Inhibitor XXI, Compound E - CAS 209986-17-4 - Calbiochem FDS
| Titre |
|---|
γ-Secretase Inhibitor XXI, Compound E - CAS 209986-17-4 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 565790 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Milano, J., et al. 2004. Toxicol. Sci. 82, 341. Jung, K.M., et al. 2003. J. Biol. Chem. 278, 42161. Murakami, D., et al. 2003. Oncogene 22, 1511. Campbell, W.A., et al. 2003. J. Neurochem. 85, 1563. Berechid, B.E., et al., 2002. J. Biol. Chem. 277, 8154. Lee, H.J., et al. 2002. J. Biol. Chem. 277, 6318. May, P., et al. 2002. J. Biol. Chem. 277, 18736. Scheinfeld, M.H., et al. 2002. J. Biol. Chem. 277, 44195. Ni, C. Y., et al. 2001. Science 294, 2179. Beher, D., et al. 2001. J. Biol. Chem. 276, 45394. Doerfler, P., et al. 2001. Proc. Natl. Acad. Sci. USA 98, 9312. Seiffert, D., et al. 2000. J. Biol. Chem. 275, 34086. |