371962 Sigma-AldrichInSolution™ H-89, Dihydrochloride - Calbiochem
InSolution™ H-89, Dihydrochloride, CAS 127243-85-0, is a 10 mM solution of H-89, 2HCl in DMSO. A cell-permeable, potent, reversible, ATP-competitive inhibitor of protein kinase A (Ki = 48 nM).
More>> InSolution™ H-89, Dihydrochloride, CAS 127243-85-0, is a 10 mM solution of H-89, 2HCl in DMSO. A cell-permeable, potent, reversible, ATP-competitive inhibitor of protein kinase A (Ki = 48 nM). Less<<Sinónimos: N-[2-((p-Bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide, 2HCl, PKA Inhibitor III
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| Empirical Formula |
|---|
| C₂₀H₂₀BrN₃O₂S |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 371962-1MG |
|
Ampolla de plást. | 1 mg |
|
— |
| Description | |
|---|---|
| Catalogue Number | 371962 |
| Brand Family | Calbiochem® |
| Synonyms | N-[2-((p-Bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide, 2HCl, PKA Inhibitor III |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | Liquid |
| Formulation | A 10 mM (1 mg/193 µl) solution of H-89, 2HCl (Cat. No. 371963) in DMSO. |
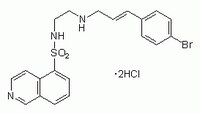
| Hill Formula | C₂₀H₂₀BrN₃O₂S |
| Chemical formula | C₂₀H₂₀BrN₃O₂S |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | PKA |
| Primary Target K<sub>i</sub> | 48 nM against protein kinase A |
| Purity | ≥99% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information | |
|---|---|
| R Phrase | R: 36/38 Irritating to eyes and skin. |
| S Phrase | S: 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 371962-1MG | 04055977213515 |
Documentation
Licencias necesarias
| Título |
|---|
| PRODUCTO REGULADO POR LA SECRETARÍA DE SALUD |
InSolution™ H-89, Dihydrochloride - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
InSolution™ H-89, Dihydrochloride - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 371962 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Leemhuis, J., et al. 2002. J. Pharmacol. Exp. Ther. 300, 1000. Davies, S.P. et al. 2000. Biochem. J. 351, 95. de Rooij, J., et al. 1998. Nature. 396, 474. Kawasaki, H., et al. 1998. Science. 282, 2275. Findik, D., et al. 1995. J. Cell. Biochem. 57, 12. Hidaka, H., and Kobayashi, R. 1992. Annu. Rev. Pharmacol. Toxicol. 32, 377. Geilen, C.C., et al. 1992. FEBS Lett. 309, 381. Chijiwa, T., et al. 1990. J. Biol. Chem. 265, 5267. Combest, W.L., et al. 1988. J. Neurochem. 51, 1581. |
| Ficha técnica | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|