375240 Sigma-AldrichHNF4 Antagonist, BI6015 - CAS 93987-29-2 - Calbiochem
The HNF4 Antagonist, BI6, also referenced under CAS 93987-29-2, controls the biological activity of HNF4.
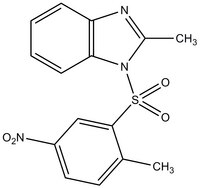
More>> The HNF4 Antagonist, BI6, also referenced under CAS 93987-29-2, controls the biological activity of HNF4. Less<<Sinónimos: 2-Methyl-1-(2-methyl-5-nitrophenylsulfonyl)-1H-benzo[d]imidazole, Hepatocyte Nuclear Factor4 Antagonist
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
| 93987-29-2 | C₁₅H₁₃N₃O₄S |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 375240-25MG |
|
Frasco de vidrio | 25 mg |
|
— |
| References | |
|---|---|
| References | Kiselyuk, A., et al. 2012. Chem. Biol. 19, 806. |
| Product Information | |
|---|---|
| CAS number | 93987-29-2 |
| Form | Beige powder |
| Hill Formula | C₁₅H₁₃N₃O₄S |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | HNFα & γ |
| Purity | ≥99% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 375240-25MG | 04055977212891 |
Documentation
HNF4 Antagonist, BI6015 - CAS 93987-29-2 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
HNF4 Antagonist, BI6015 - CAS 93987-29-2 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 375240 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Kiselyuk, A., et al. 2012. Chem. Biol. 19, 806. |