533659 Sigma-AldrichERRα Inverse Agonist, C29 - Calbiochem
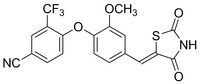
ERRα Inverse Agonist, C29, is a cell-permeable, potent, time-dependent, and slowly reversible inhibitor of ERRα activity (IC₅₀ = 40 and 600 nM in TR-FRET and two-hybrid luciferase reporter assay).
More>> ERRα Inverse Agonist, C29, is a cell-permeable, potent, time-dependent, and slowly reversible inhibitor of ERRα activity (IC₅₀ = 40 and 600 nM in TR-FRET and two-hybrid luciferase reporter assay). Less<<Sinónimos: (Z)-4-(4-((2,4-Dioxothiazolidin-5-ylidene)methyl)-2-methoxyphenoxy)-3-(trifluoromethyl)benzonitrile, Estrogen-Related Receptor α Inverse Agonist, NR3B1 Inverse Agonist
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| Empirical Formula |
|---|
| C₁₉H₁₁F₃N₂O₄S |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 5.33659.0001 |
|
Frasco de vidrio | 10 mg |
|
— |
| References | |
|---|---|
| References | Chaveroux, C., et al. 2013. Cell Metab. 17, 586. Patch, R.J., et al. 2011. J. Med. Chem. 54, 788. |
| Product Information | |
|---|---|
| Form | Pale yellow solid |
| Hill Formula | C₁₉H₁₁F₃N₂O₄S |
| Chemical formula | C₁₉H₁₁F₃N₂O₄S |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | ERRα |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 5.33659.0001 | 04055977286595 |
Documentation
ERRα Inverse Agonist, C29 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Referencias bibliográficas
| Visión general referencias |
|---|
| Chaveroux, C., et al. 2013. Cell Metab. 17, 586. Patch, R.J., et al. 2011. J. Med. Chem. 54, 788. |
| Ficha técnica | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|