324380 Sigma-AldrichDoxorubicin, Hydrochloride - CAS 25316-40-9 - Calbiochem
Doxorubicin HCl, CAS 25316-40-9, is an antitumor antibiotic that inhibits topoisomerase II (IC₅₀ = 100 nM).
More>> Doxorubicin HCl, CAS 25316-40-9, is an antitumor antibiotic that inhibits topoisomerase II (IC₅₀ = 100 nM). Less<<Sinónimos: Adriamycin, 14-Hydroxydaunomycin, HCl
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
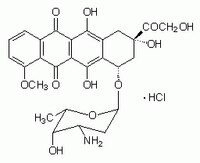
| 25316-40-9 | C₂₇H₂₉NO₁₁ · HCl |
| Description | |
|---|---|
| Overview | Antitumor antibiotic and a highly effective myotoxin that inhibits topoisomerase II (IC50 = 100 nM). Binds to nucleic acids, presumably by specific intercalation into the DNA double helix, thereby inhibiting nucleic acid synthesis. Induces apoptosis in rhabdomyosarcoma cell lines. Also available as a 10 mM solution in H2O (Cat. No. 504042). |
| Catalogue Number | 324380 |
| Brand Family | Calbiochem® |
| Synonyms | Adriamycin, 14-Hydroxydaunomycin, HCl |
| Product Information | |
|---|---|
| CAS number | 25316-40-9 |
| ATP Competitive | N |
| Form | Orange to red powder |
| Hill Formula | C₂₇H₂₉NO₁₁ · HCl |
| Chemical formula | C₂₇H₂₉NO₁₁ · HCl |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | topoisomerase 2 |
| Primary Target IC<sub>50</sub> | 100 nM |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | QI9295900 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 324380 | 0 |
Documentation
Doxorubicin, Hydrochloride - CAS 25316-40-9 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Doxorubicin, Hydrochloride - CAS 25316-40-9 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 324380 |
Referencias bibliográficas
| Visión general referencias |
|---|
| A'Hern, R.P., and Gore, M.E. 1995. J. Clin. Oncol. 13, 726. Nooter, K., et al. 1995. Br. J. Cancer 71, 556. Noviello, E., et al. 1994. Mutat. Res. 311, 21. Anderson, R.D., et al. 1993. Mutat. Res. 294, 215. Hershko, C., et al. 1993. J. Lab. Clin. Med. 122, 245. Jongmans, W., et al. 1993. Mutat. Res. 294, 207. O'Shaughnessy, J.A., and Cowan, K.H. 1993. J. Am. Med. Assoc. 270, 2089. Theyer, G., et al. 1993. J. Urol. 150, 1544. Tritton, T.R., and Yee, G. 1982. Science 217, 248. |
Folleto
| Cargo |
|---|
| Tools and Tips for Analyzing Apoptosis |