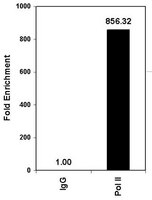
Altered nucleosome positioning at the transcription start site and deficient transcriptional initiation in Friedreich ataxia.
Chutake, YK; Costello, WN; Lam, C; Bidichandani, SI
The Journal of biological chemistry
289
15194-202
2014
Mostrar resumen
Most individuals with Friedreich ataxia (FRDA) are homozygous for an expanded GAA triplet repeat (GAA-TR) mutation in intron 1 of the FXN gene, which results in deficiency of FXN transcript. Consistent with the expanded GAA-TR sequence as a cause of variegated gene silencing, evidence for heterochromatin has been detected in intron 1 in the immediate vicinity of the expanded GAA-TR mutation in FRDA. Transcriptional deficiency in FRDA is thought to result from deficient elongation through the expanded GAA-TR sequence because of repeat-proximal heterochromatin and abnormal DNA structures adopted by the expanded repeat. There is also evidence for deficient transcriptional initiation in FRDA, but its relationship to the expanded GAA-TR mutation remains unclear. We show that repressive chromatin extends from the expanded GAA-TR in intron 1 to the upstream regions of the FXN gene, involving the FXN transcriptional start site. Using a chromatin accessibility assay and a high-resolution nucleosome occupancy assay, we found that the major FXN transcriptional start site, which is normally in a nucleosome-depleted region, is rendered inaccessible by altered nucleosome positioning in FRDA. Consistent with the altered epigenetic landscape the FXN gene promoter, a typical CpG island promoter, was found to be in a transcriptionally non-permissive state in FRDA. Both metabolic labeling of nascent transcripts and an unbiased whole transcriptome analysis revealed a severe deficiency of transcriptional initiation in FRDA. Deficient transcriptional initiation, and not elongation, is the major cause of FXN transcriptional deficiency in FRDA, and it is related to the spread of repressive chromatin from the expanded GAA-TR mutation. | 24737321
 |
The p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal transition in human bladder cancer cells: role of MIR-205.
Tran, MN; Choi, W; Wszolek, MF; Navai, N; Lee, IL; Nitti, G; Wen, S; Flores, ER; Siefker-Radtke, A; Czerniak, B; Dinney, C; Barton, M; McConkey, DJ
The Journal of biological chemistry
288
3275-88
2013
Mostrar resumen
Epithelial-mesenchymal transition (EMT) is a physiological process that plays important roles in tumor metastasis, "stemness," and drug resistance. EMT is typically characterized by the loss of the epithelial marker E-cadherin and increased expression of EMT-associated transcriptional repressors, including ZEB1 and ZEB2. The miR-200 family and miR-205 prevent EMT through suppression of ZEB1/2. p53 has been implicated in the regulation of miR-200c, but the mechanisms controlling miR-205 expression remain elusive. Here we report that the p53 family member and p63 isoform, ΔNp63α, promotes miR-205 transcription and controls EMT in human bladder cancer cells. ΔNp63α, E-cadherin and miR-205 were coexpressed in a panel of bladder cancer cell lines (n = 28) and a cohort of primary bladder tumors (n = 98). Stable knockdown of ΔNp63α in the "epithelial" bladder cancer cell line UM-UC6 decreased the expression of miR-205 and induced the expression of ZEB1/2, effects that were reversed by expression of exogenous miR-205. Conversely, overexpression of ΔNp63α in the "mesenchymal" bladder cancer cell line UM-UC3 induced miR-205 and suppressed ZEB1/2. ΔNp63α knockdown reduced the expression of the primary and mature forms of miR-205 and the miR-205 "host" gene (miR-205HG) and decreased binding of RNA Pol II to the miR-205HG promoter, inhibiting miR-205HG transcription. Finally, high miR-205 expression was associated with adverse clinical outcomes in bladder cancer patients. Together, our data demonstrate that ΔNp63α-mediated expression of miR-205 contributes to the regulation of EMT in bladder cancer cells and identify miR-205 as a molecular marker of the lethal subset of human bladder cancers. | 23239884
 |
Rbfox1 downregulation and altered calpain 3 splicing by FRG1 in a mouse model of Facioscapulohumeral muscular dystrophy (FSHD).
Pistoni, M; Shiue, L; Cline, MS; Bortolanza, S; Neguembor, MV; Xynos, A; Ares, M; Gabellini, D
PLoS genetics
9
e1003186
2013
Mostrar resumen
Facioscapulohumeral muscular dystrophy (FSHD) is a common muscle disease whose molecular pathogenesis remains largely unknown. Over-expression of FSHD region gene 1 (FRG1) in mice, frogs, and worms perturbs muscle development and causes FSHD-like phenotypes. FRG1 has been implicated in splicing, and we asked how splicing might be involved in FSHD by conducting a genome-wide analysis in FRG1 mice. We find that splicing perturbations parallel the responses of different muscles to FRG1 over-expression and disease progression. Interestingly, binding sites for the Rbfox family of splicing factors are over-represented in a subset of FRG1-affected splicing events. Rbfox1 knockdown, over-expression, and RNA-IP confirm that these are direct Rbfox1 targets. We find that FRG1 is associated to the Rbfox1 RNA and decreases its stability. Consistent with this, Rbfox1 expression is down-regulated in mice and cells over-expressing FRG1 as well as in FSHD patients. Among the genes affected is Calpain 3, which is mutated in limb girdle muscular dystrophy, a disease phenotypically similar to FSHD. In FRG1 mice and FSHD patients, the Calpain 3 isoform lacking exon 6 (Capn3 E6-) is increased. Finally, Rbfox1 knockdown and over-expression of Capn3 E6- inhibit muscle differentiation. Collectively, our results suggest that a component of FSHD pathogenesis may arise by over-expression of FRG1, reducing Rbfox1 levels and leading to aberrant expression of an altered Calpain 3 protein through dysregulated splicing. | 23300487
 |
A SALL4/MLL/HOXA9 pathway in murine and human myeloid leukemogenesis.
Li, A; Yang, Y; Gao, C; Lu, J; Jeong, HW; Liu, BH; Tang, P; Yao, X; Neuberg, D; Huang, G; Tenen, DG; Chai, L
The Journal of clinical investigation
123
4195-207
2013
Mostrar resumen
The embryonic self-renewal factor SALL4 has been implicated in the development of human acute myeloid leukemia (AML). Transgenic mice expressing the human SALL4B allele develop AML, which indicates that this molecule contributes to leukemia development and maintenance. However, the underlying mechanism of SALL4-dependent AML progression is unknown. Using SALL4B transgenic mice, we observed that HoxA9 was significantly upregulated in SALL4B leukemic cells compared with wild-type controls. Downregulation of HoxA9 in SALL4B leukemic cells led to decreased replating capacity in vitro and delayed AML development in recipient mice. In primary human AML cells, downregulation of SALL4 led to decreased HOXA9 expression and enhanced apoptosis. We found that SALL4 bound a specific region of the HOXA9 promoter in leukemic cells. SALL4 overexpression led to enhanced binding of histone activation markers at the HOXA9 promoter region, as well as increased HOXA9 expression in these cells. Furthermore, we observed that SALL4 interacted with mixed-lineage leukemia (MLL) and co-occupied the HOXA9 promoter region with MLL in AML leukemic cells, which suggests that a SALL4/MLL pathway may control HOXA9 expression. In summary, our findings revealed a molecular mechanism for SALL4 function in leukemogenesis and suggest that targeting of the SALL4/MLL/HOXA9 pathway would be an innovative approach in treating AML. | 24051379
 |
Abnormal histone methylation is responsible for increased vascular endothelial growth factor 165a secretion from airway smooth muscle cells in asthma.
Clifford, RL; John, AE; Brightling, CE; Knox, AJ
Journal of immunology (Baltimore, Md. : 1950)
189
819-31
2011
Mostrar resumen
Vascular endothelial growth factor (VEGF), a key angiogenic molecule, is aberrantly expressed in several diseases including asthma where it contributes to bronchial vascular remodeling and chronic inflammation. Asthmatic human airway smooth muscle cells hypersecrete VEGF, but the mechanism is unclear. In this study, we defined the mechanism in human airway smooth muscle cells from nonasthmatic and asthmatic patients. We found that asthmatic cells lacked a repression complex at the VEGF promoter, which was present in nonasthmatic cells. Recruitment of G9A, trimethylation of histone H3 at lysine 9 (H3K9me3), and a resultant decrease in RNA polymerase II at the VEGF promoter was critical to repression of VEGF secretion in nonasthmatic cells. At the asthmatic promoter, H3K9me3 was absent because of failed recruitment of G9a; RNA polymerase II binding, in association with TATA-binding protein-associated factor 1, was increased; H3K4me3 was present; and Sp1 binding was exaggerated and sustained. In contrast, DNA methylation and histone acetylation were similar in asthmatic and nonasthmatic cells. This is the first study, to our knowledge, to show that airway cells in asthma have altered epigenetic regulation of remodeling gene(s). Histone methylation at genes such as VEGF may be an important new therapeutic target. | 22689881
 |
Discovery of SMAD4 promoters, transcription factor binding sites and deletions in juvenile polyposis patients.
Calva, D; Dahdaleh, FS; Woodfield, G; Weigel, RJ; Carr, JC; Chinnathambi, S; Howe, JR
Nucleic acids research
39
5369-78
2010
Mostrar resumen
Inactivation of SMAD4 has been linked to several cancers and germline mutations cause juvenile polyposis (JP). We set out to identify the promoter(s) of SMAD4, evaluate their activity in cell lines and define possible transcription factor binding sites (TFBS). 5'-rapid amplification of cDNA ends (5'-RACE) and computational analyses were used to identify candidate promoters and corresponding TFBS and the activity of each was assessed by luciferase vectors in different cell lines. TFBS were disrupted by site-directed mutagenesis (SDM) to evaluate the effect on promoter activity. Four promoters were identified, two of which had significant activity in several cell lines, while two others had minimal activity. In silico analysis revealed multiple potentially important TFBS for each promoter. One promoter was deleted in the germline of two JP patients and SDM of several sites led to significant reduction in promoter activity. No mutations were found by sequencing this promoter in 65 JP probands. The predicted TFBS profiles for each of the four promoters shared few transcription factors in common, but were conserved across several species. The elucidation of these promoters and identification of TFBS has important implications for future studies in sporadic tumors from multiple sites, and in JP patients. Artículo Texto completo | 21421563
 |