189510 Sigma-AldrichAutotaxin Inhibitor I, S32826 - CAS 1103672-43-0 - Calbiochem
The Autotaxin Inhibitor I, S32826, also referenced under CAS 1103672-43-0, controls the biological activity of Autotaxin.
More>> The Autotaxin Inhibitor I, S32826, also referenced under CAS 1103672-43-0, controls the biological activity of Autotaxin. Less<<Sinónimos: ATX Inhibitor I, (4-(Tetradecanoylamino)benzyl)phosphonic acid disodium, dihydrate
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
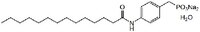
| 1103672-43-0 | C₂₁H₃₄NO₄PNa₂ • 2H₂O |
Precios y disponibilidad
| Número de referencia | Disponiblidad | Embalaje | Cant./Env. | Precio | Cantidad | |
|---|---|---|---|---|---|---|
| 189510-5MG |
|
Frasco de vidrio | 5 mg |
|
— |
| Description | |
|---|---|
| Overview | A phosphonate compound that potently inhibits both the phosphodiesterase (IC50 = 9 nM in pNppp assay using ATX β) and the lysoPLD (IC50 = 5.6 nM and 47 nM, by enzyme-linked fluorescence detection or autoradiography by TLC, respectively, using ATX β) activities of ATX α/β/γ, while exhibiting much reduced (IC50 ~ 6 µM and 15 µM against Src and PTP-1B, respectively) or little activity toward 29 other receptors and enzymes. Shown to block LPA release from murine 3T3F422A adipocytes (IC50 = 90 nM) in vitro and from excised human and rat adipose tissues (by ~70% inhibition at 0.2 and 1 µM, respectively) in cultures ex vivo. The in vivo applicability of S32826 is limited by its low epithelial permeability and other poor pharmacokinetic properties. CTAB (Cat. No. 219374) is reported to counteract the inhibitory activity of S32826, 2-methyl-2,4-pentanediol can be used instead to stabilize purified enzyme in solution during S32826 inhibition assays. |
| Catalogue Number | 189510 |
| Brand Family | Calbiochem® |
| Synonyms | ATX Inhibitor I, (4-(Tetradecanoylamino)benzyl)phosphonic acid disodium, dihydrate |
| References | |
|---|---|
| References | Boutin, J.A., and Ferry, G. 2009. Cell Mol. Life Sci. 66, 3009. Samadi, N., et al. 2009. Oncogene 28, 1028. Ferry, G., et al. 2008. J. Pharm. Exp. Ther. 327, 809. |
| Product Information | |
|---|---|
| CAS number | 1103672-43-0 |
| Form | White powder |
| Hill Formula | C₂₁H₃₄NO₄PNa₂ • 2H₂O |
| Chemical formula | C₂₁H₃₄NO₄PNa₂ • 2H₂O |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 189510-5MG | 04055977221862 |
Documentation
Autotaxin Inhibitor I, S32826 - CAS 1103672-43-0 - Calbiochem Ficha datos de seguridad (MSDS)
| Título |
|---|
Autotaxin Inhibitor I, S32826 - CAS 1103672-43-0 - Calbiochem Certificados de análisis
| Cargo | Número de lote |
|---|---|
| 189510 |
Referencias bibliográficas
| Visión general referencias |
|---|
| Boutin, J.A., and Ferry, G. 2009. Cell Mol. Life Sci. 66, 3009. Samadi, N., et al. 2009. Oncogene 28, 1028. Ferry, G., et al. 2008. J. Pharm. Exp. Ther. 327, 809. |