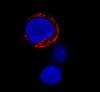
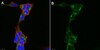
189412 Aurora Kinase Inhibitor XI, HOI-07
Sinónimos: (E)-3-((E)-4-(Benzo[d][1,3]dioxol-5-yl)-2-oxobut-3-enylidene)indolin-2-one
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| Empirical Formula |
|---|
| C₁₉H₁₃NO₄ |
| References | |
|---|---|
| References | Xie, H., et al. 2012. Cancer Res. 73, 716. |
| Product Information | |
|---|---|
| Form | Red solid |
| Hill Formula | C₁₉H₁₃NO₄ |
| Chemical formula | C₁₉H₁₃NO₄ |
| Reversible | Y |
| Structure formula Image | |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Aurora B |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 189412 | 0 |
Documentation
Referencias bibliográficas
| Visión general referencias |
|---|
| Xie, H., et al. 2012. Cancer Res. 73, 716. |