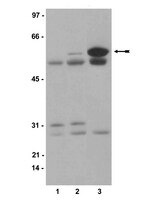
FAK inhibition abrogates the malignant phenotype in aggressive pediatric renal tumors.
Megison, ML; Gillory, LA; Stewart, JE; Nabers, HC; Mrozcek-Musulman, E; Beierle, EA
Molecular cancer research : MCR
12
514-26
2014
Mostrar resumen
Despite the tremendous advances in the treatment of childhood kidney tumors, there remain subsets of pediatric renal tumors that continue to pose a therapeutic challenge, mainly malignant rhabdoid kidney tumors and nonosseous renal Ewing sarcoma. Children with advanced, metastatic, or relapsed disease have a poor disease-free survival rate. Focal adhesion kinase (FAK) is a nonreceptor tyrosine kinase that is important in many facets of tumor development and progression. FAK has been found in other pediatric solid tumors and in adult renal cellular carcinoma, leading to the hypothesis that FAK contributes to pediatric kidney tumors and would affect cellular survival. In the current study, FAK was present and phosphorylated in pediatric kidney tumor specimens. Moreover, the effects of FAK inhibition upon G401 and SK-NEP-1 cell lines were examined using a number of parallel approaches to block FAK, including RNA interference and small-molecule FAK inhibitors. FAK inhibition resulted in decreased cellular survival, invasion and migration, and increased apoptosis. Furthermore, small-molecule inhibition of FAK led to decreased SK-NEP-1 xenograft growth in vivo. These data deepen the knowledge of the tumorigenic process in pediatric renal tumors, and provide desperately needed therapeutic strategies and targets for these rare, but difficult to treat, malignancies.This study provides a fundamental understanding of tumorigenesis in difficult to treat renal tumors and provides an impetus for new avenues of research and potential for novel, targeted therapies. | | 24464916
 |
Individual Src-family tyrosine kinases direct the degradation or protection of the clock protein Timeless via differential ubiquitylation.
O'Reilly, LP; Zhang, X; Smithgall, TE
Cellular signalling
25
860-6
2013
Mostrar resumen
Timeless was originally identified in Drosophila as an essential component of circadian cycle regulation, where its function is tightly controlled at the protein level by tyrosine phosphorylation and subsequent degradation. In mammals, Timeless has also been implicated in circadian rhythms as well as cell cycle control and embryonic development. Here we report that mammalian Timeless is an SH3 domain-binding protein and substrate for several members of the Src protein-tyrosine kinase family, including Fyn, Hck, c-Src and c-Yes. Co-expression of Tim with Fyn or Hck was followed by ubiquitylation and subsequent degradation in human 293T cells. While c-Src and c-Yes also promoted Tim ubiquitylation, in this case ubiquitylation correlated with Tim protein accumulation rather than degradation. Both c-Src and c-Yes selectively promoted modification of Tim through Lys63-linked polyubiquitin, which may explain the differential effects on Tim protein turnover. These data show distinct and opposing roles for individual Src-family members in the regulation of Tim protein levels, suggesting a unique mechanism for the regulation of Tim function in mammals. | | 23266470
 |
Intercellular adhesion molecule-2 is involved in apical ectoplasmic specialization dynamics during spermatogenesis in the rat.
Xiao, X; Cheng, CY; Mruk, DD
The Journal of endocrinology
216
73-86
2013
Mostrar resumen
In this study, we investigated the role of intercellular adhesion molecule-2 (ICAM2) in the testis. ICAM2 is a cell adhesion protein having important roles in cell migration, especially during inflammation when leukocytes cross the endothelium. Herein, we showed ICAM2 to be expressed by germ and Sertoli cells in the rat testis. When a monospecific antibody was used for immunolocalization experiments, ICAM2 was found to surround the heads of elongating/elongated spermatids in all stages of the seminiferous epithelial cycle. To determine whether ICAM2 is a constituent of apical ectoplasmic specialization (ES), co-immunoprecipitation and dual immunofluorescence staining were performed. Interestingly, ICAM2 was found to associate with β1-integrin, nectin-3, afadin, Src, proline-rich tyrosine kinase 2, annexin II, and actin. Following CdCl₂ treatment, ICAM2 was found to be upregulated during restructuring of the seminiferous epithelium, with round spermatids becoming increasingly immunoreactive for ICAM2 by 6-16 h. Interestingly, there was a loss in the binding of ICAM2 to actin during CdCl₂-induced germ cell loss, suggesting that a loss of ICAM2-actin interactions might have facilitated junction restructuring. Taken collectively, these results illustrate that ICAM2 plays an important role in apical ES dynamics during spermatogenesis. | | 23097088
 |
Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis.
Koreckij, T; Nguyen, H; Brown, LG; Yu, EY; Vessella, RL; Corey, E
British journal of cancer
101
263-8
2009
Mostrar resumen
Dasatinib is a small molecule kinase inhibitor that has recently been shown to inhibit Src family kinases (SFK) and also has activity against CaP. Of importance to metastatic CaP, which frequently metastasises to bone, SFK are also vital to the regulation of bone remodelling. We sought to determine the ability of dasatinib to inhibit growth of CaP in bone.C4-2B CaP cells were injected into tibiae of SCID mice and treated with dasatinib, alone or in combination with docetaxel. Serum prostate-specific antigen levels, bone mineral density, radiographs and histology were analysed.Treatment with dasatinib alone significantly lowered sacrifice serum prostate-specific antigen levels compared to control, 2.3+/-0.4 vs 9.2+/-2.1 (P=0.004). Combination therapy improved efficacy over dasatinib alone (P=0.010). Dasatinib increased bone mineral density in tumoured tibiae by 25% over control tumoured tibiae (Pless than 0.001).Dasatinib inhibits growth of C4-2B cells in bone with improved efficacy when combined with docetaxel. Additionally, dasatinib inhibits osteolysis associated with CaP. These data support further study of dasatinib in clinical trials for men with CaP bone metastases. Artículo Texto completo | | 19603032
 |
Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium.
Lie, PP; Mruk, DD; Lee, WM; Cheng, CY
FASEB journal : official publication of the Federation of American Societies for Experimental Biology
23
2555-67
2009
Mostrar resumen
In the seminiferous epithelium, Eps8 is localized to actin-based cell junctions at the blood-testis barrier (BTB) and the apical ectoplasmic specialization (ES) in stage V-VI tubules but is considerably diminished in stage VIII tubules. Eps8 down-regulation coincides with the time of BTB restructuring and apical ES disassembly, implicating the role of Eps8 in cell adhesion. Its involvement in Sertoli-germ cell adhesion was substantiated in studies using an in vivo animal model by treating rats with 1-(2,4-dichlorobenzy)-1H-indazole-3-carbohydrazide (adjudin) to induce anchoring junction restructuring, during which Eps8 disappeared at the apical ES before germ cell departure. In Sertoli cell cultures with established permeability barrier mimicking the BTB in vivo, the knockdown of Eps8 by RNAi led to F-actin disorganization and the mislocalization of the tight junction proteins occludin and ZO-1, suggesting the function of Eps8 in maintaining BTB integrity. In vivo knockdown of Eps8 in the testis caused germ cell sloughing and BTB damage, concomitant with occludin mislocalization, further validating that Eps8 is a novel regulator of cell adhesion and BTB integrity in the seminiferous epithelium. | | 19293393
 |
Mechanism of the vascular angiotensin II/alpha2-adrenoceptor interaction.
Jackson, EK; Gao, L; Zhu, C
The Journal of pharmacology and experimental therapeutics
314
1109-16
2004
Mostrar resumen
alpha(2)-Adrenoceptors potentiate vascular responses to angiotensin II. The goal of this study was to test the hypothesis that the phospholipase C (PLC)/protein kinase C (PKC)/c-src/phosphatidylinositol 3-kinase (PI3K) pathway contributes to the vascular angiotensin II/alpha(2)-adrenoceptor interaction. In rats in vivo, intrarenal infusions of angiotensin II (10 ng/kg/min) increased renal vascular resistance by 5.8 +/- 0.5 units, and this response was enhanced (p less than 0.05) to 9.1 +/- 1.2 units by UK-14,304 [5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine; 3 microg/kg/min; alpha(2)-adrenoceptor agonist]. Intrarenal infusions of U-73122 [1-[6-[[(17beta)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]-hexyl]-1H-pyrrole-2,5-dione; 3 microg/min; PLC inhibitor], GF109203X [bisindolylmaleimide I; 10 microg/min; PKC inhibitor], CGP77675 [1-(2-{4-[4-amino-5-(3-methoxyphenyl)pyrrolo[2,3-d]pyrimidin-7-yl]phenyl}ethyl)piperidin-4-ol; 5 microg/min; c-src inhibitor], and wortmannin (1 microg/min; PI3K inhibitor) abolished the angiotensin II/alpha(2)-adrenoceptor interaction. In isolated perfused rat kidneys, angiotensin II (0.3, 1, and 3 nM) increased perfusion pressure (by 15 +/- 8, 39 +/- 4, and 93 +/- 9 mm Hg, respectively), and UK-14,304 (1 microM) potentiated these responses (to 36 +/- 4, 67 +/- 7, and 135 +/- 17 mm Hg, respectively). This angiotensin II/alpha(2)-adrenoceptor interaction was abolished by U-73122 (10 microM), GF109203X (3 microM), CGP77675 (5 microM), and wortmannin (0.2 microM). Preglomerular microvascular smooth muscle cells expressed phospholipase (PLC)-beta(2), PLC-beta(3), c-src, phospho(tyrosine 416)-c-src, and PI3K. In these cells, angiotensin II (0.1 microM) and UK-14,304 (1 microM) per se did not increase phospho-c-src; however, the combination of angiotensin II plus UK-14,304 doubled phospho-c-src, and this interaction was abolished by U-73122 (10 microM) and GF109203X (3 microM). In conclusion, the PLC/PKC/c-src/PI3K pathway may contribute importantly to the interaction between alpha(2)-adrenoceptors and angiotensin II on renal vascular resistance. | | 15901799
 |
Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes.
Wong, CH; Xia, W; Lee, NP; Mruk, DD; Lee, WM; Cheng, CY
Endocrinology
146
1192-204
2004
Mostrar resumen
Apical ectoplasmic specialization (ES) is a unique testis-specific cell-cell actin-based adherens junction type restricted to the Sertoli-round/elongating/elongate spermatid interface in the seminiferous epithelium. An endogenous testosterone (T) suppression model was used to study the regulation of apical ES dynamics in the testis. By providing sustained releases of T and estradiol using subdermal implants in rats, this treatment reduced endogenous testicular T level. This in turn led to sloughing of spermatids (step 8 and beyond) from the seminiferous epithelium, which can be reversed by removing the implants, or replacing them with a higher dose of T implants. This model thus allows us to study the restructuring events at the apical ES. It was shown that apical ES restructuring involved proteins that were usually restricted to the cell-matrix focal adhesion site in other epithelia. For instance, the protein levels of beta1-integrin, Tyr-phosphorylated focal adhesion kinase (p-FAK), and c-Src were induced during the T suppression-induced germ cell loss and recovery, implicating that these proteins are putative regulators of ES dynamics. Indeed, the formation of p-FAK/c-Src protein complex, but not their association with beta1-integrin, was stimulated during T suppression-induced germ cell loss. ERK, a MAPK known to regulate focal adhesion turnover, was also activated during the androgen suppression-induced spermatid loss and the early phase of the recovery when germ cells began to repopulate the epithelium. Collectively, these data suggest that the apical ES is a cell-cell adherens junction type with the characteristics of cell-matrix focal contacts. In addition to its role in conferring cell adhesion formation, the p-FAK/c-Src protein complex apparently also regulates apical ES disruption via the ERK signaling pathway. | Immunofluorescence | 15591141
 |
Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation.
Jacob B Hansen, Claus Jørgensen, Rasmus K Petersen, Philip Hallenborg, Rita De Matteis, Hans A Bøye, Natasa Petrovic, Sven Enerbäck, Jan Nedergaard, Saverio Cinti, Hein te Riele, Karsten Kristiansen
Proceedings of the National Academy of Sciences of the United States of America
101
4112-7
2004
Mostrar resumen
Adipocyte precursor cells give raise to two major cell populations with different physiological roles: white and brown adipocytes. Here we demonstrate that the retinoblastoma protein (pRB) regulates white vs. brown adipocyte differentiation. Functional inactivation of pRB in wild-type mouse embryo fibroblasts (MEFs) and white preadipocytes by expression of simian virus 40 large T antigen results in the expression of the brown fat-specific uncoupling protein 1 (UCP-1) in the adipose state. Retinoblastoma gene-deficient (Rb-/-) MEFs and stem cells, but not the corresponding wild-type cells, differentiate into adipocytes with a gene expression pattern and mitochondria content resembling brown adipose tissue. pRB-deficient MEFs exhibit an increased expression of the Forkhead transcription factor Foxc2 and its target gene cAMP-dependent protein kinase regulatory subunit RIalpha, resulting in increased cAMP sensitivity. Suppression of cAMP-dependent protein kinase activity in Rb(-/-)MEFs blocked the brown adipocyte-like gene expression pattern without affecting differentiation per se. Immunohistochemical studies revealed that pRB is present in the nuclei of white but not brown adipocyte precursor cells at a developmental stage where both cell types begin to accumulate lipid and brown adipocytes express UCP-1. Furthermore, pRB rapidly undergoes phosphorylation upon cold-induced neodifferentiation and up-regulation of UCP-1 expression in brown adipose tissue. Finally, down-regulation of pRB expression accompanies transdifferentiation of white into brown adipocytes in response to beta3-adrenergic receptor agonist treatment. We propose that pRB acts as a molecular switch determining white vs. brown adipogenesis, suggesting a previously uncharacterized function of this key cell cycle regulator in adipocyte lineage commitment and differentiation. Artículo Texto completo | | 15024128
 |
Alternative splicing modulates Disabled-1 (Dab1) function in the developing chick retina
Katyal, S. and Godbout, R.
Embo J, 23:1878-88 (2004)
2004
| Immunocytochemistry | 15057276
 |