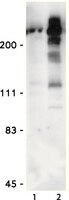
Prdx4 is a compartment-specific H2O2 sensor that regulates neurogenesis by controlling surface expression of GDE2.
Yan, Y; Wladyka, C; Fujii, J; Sockanathan, S
Nature communications
6
7006
2015
Mostrar resumen
Neural progenitors and terminally differentiated neurons show distinct redox profiles, suggesting that coupled-redox cascades regulate the initiation and progression of neuronal differentiation. Discrete cellular compartments have different redox environments and how they contribute to differentiation is unclear. Here we show that Prdx4, an endoplasmic reticulum (ER) enzyme that metabolizes H2O2, acts as a tunable regulator of neurogenesis via its compartmentalized thiol-oxidative function. Prdx4 ablation causes premature motor neuron differentiation and progenitor depletion, leading to imbalances in subtype-specific motor neurons. GDE2, a six-transmembrane protein that induces differentiation by downregulating Notch signalling through surface cleavage of GPI-anchored proteins, is targeted by Prdx4 oxidative activity. Prdx4 dimers generated by H2O2 metabolism oxidize two cysteine residues within the GDE2 enzymatic domain, which blocks GDE2 trafficking to the plasma membrane and prevents GDE2 neurogeneic function. Thus, Prdx4 oxidative activity acts as a sensor to directly couple neuronal differentiation with redox environments in the ER. | | 25943695
 |
Cdk1 phosphorylates the Rac activator Tiam1 to activate centrosomal Pak and promote mitotic spindle formation.
Whalley, HJ; Porter, AP; Diamantopoulou, Z; White, GR; Castañeda-Saucedo, E; Malliri, A
Nature communications
6
7437
2015
Mostrar resumen
Centrosome separation is critical for bipolar spindle formation and the accurate segregation of chromosomes during mammalian cell mitosis. Kinesin-5 (Eg5) is a microtubule motor essential for centrosome separation, and Tiam1 and its substrate Rac antagonize Eg5-dependent centrosome separation in early mitosis promoting efficient chromosome congression. Here we identify S1466 of Tiam1 as a novel Cdk1 site whose phosphorylation is required for the mitotic function of Tiam1. We find that this phosphorylation of Tiam1 is required for the activation of group I p21-activated kinases (Paks) on centrosomes in prophase. Further, we show that both Pak1 and Pak2 counteract centrosome separation in a kinase-dependent manner and demonstrate that they act downstream of Tiam1. We also show that depletion of Pak1/2 allows cells to escape monopolar arrest by Eg5 inhibition, highlighting the potential importance of this signalling pathway for the development of Eg5 inhibitors as cancer therapeutics. | | 26078008
 |
Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism.
Yaffe, M B, et al.
Science, 278: 1957-60 (1997)
1997
Mostrar resumen
Pin1 is an essential and conserved mitotic peptidyl-prolyl isomerase (PPIase) that is distinct from members of two other families of conventional PPIases, cyclophilins and FKBPs (FK-506 binding proteins). In response to their phosphorylation during mitosis, Pin1 binds and regulates members of a highly conserved set of proteins that overlaps with antigens recognized by the mitosis-specific monoclonal antibody MPM-2. Pin1 is here shown to be a phosphorylation-dependent PPIase that specifically recognizes the phosphoserine-proline or phosphothreonine-proline bonds present in mitotic phosphoproteins. Both Pin1 and MPM-2 selected similar phosphorylated serine-proline-containing peptides, providing the basis for the specific interaction between Pin1 and MPM-2 antigens. Pin1 preferentially isomerized proline residues preceded by phosphorylated serine or threonine with up to 1300-fold selectivity compared with unphosphorylated peptides. Pin1 may thus regulate mitotic progression by catalyzing sequence-specific and phosphorylation-dependent proline isomerization. | Immunoblotting (Western) | 9395400
 |
Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope.
Westendorf, J M, et al.
Proc. Natl. Acad. Sci. U.S.A., 91: 714-8 (1994)
1993
Mostrar resumen
The MPM2 monoclonal antibody binds to a phospho amino acid-containing epitope present on more than 40 proteins of M-phase eukaryotic cells. We have developed a technique for cloning cDNAs encoding MPM2-reactive phosphoproteins from bacteriophage lambda expression libraries. Proteins from phage plaques were absorbed to nitrocellulose filters, phosphorylated by M-phase kinases, and screened for MPM2 binding. Partial-length cDNAs encoding two MPM2-reactive proteins termed MPM2-reactive phosphoproteins 1 and 2 (MPP1 and MPP2) were isolated. The deduced MPP1 and MPP2 amino acid sequences are not closely related to any previously described proteins. To determine which amino acid stretches contained the MPM2 epitope, sequences from a 15 amino acid peptide expression library were selected for binding to MPM2 after phosphorylation by M-phase kinases. A string of five amino acids was similar among all selected peptides, and the sequence reflecting the most frequent amino acid at each position was Leu-Thr-Pro-Leu-Lys (LTPLK). MPP1 and MPP2 proteins, respectively, contained five and nine sites closely related to LTPLK, including two that were common to both proteins, (F/T)TPLQ and SSP(I/S)D. Peptides containing LTPLK and FTPLQ were strongly phosphorylated by M-phase, but not interphase, cytosolic kinases, and the phosphorylated peptides were bound by MPM2. Thus, we have identified M-phase-specific phosphorylation sites bound by MPM2 and two putative M-phase phosphoproteins containing these sites. | | 8290587
 |
The mitosis-specific monoclonal antibody MPM-2 recognizes phosphoproteins associated with the nuclear envelope in Chlamydomonas reinhardtii cells.
Harper, J D, et al.
Eur. J. Cell Biol., 51: 272-8 (1990)
1990
Mostrar resumen
The monoclonal antibody MPM-2 recognizes a family of phosphorylated proteins present in mitotic cells. In a number of organisms it stains nuclei and also cytoskeletal structures which contain or organize tubulin. In mitotic Chlamydomonas reinhardtii cells MPM-2 reacts with phosphoproteins associated with the nuclear envelope (NE). Staining of the NE region appears in preprophase, reaches a maximum intensity in metaphase/anaphase and disappears rapidly in telophase. Localized hyperphosphorylation of the anterior NE region is apparent in many cells throughout mitosis. The distribution and timing of MPM-2 labeling suggests that in Chlamydomonas MPM-2 may be interacting with lamin-like phosphoproteins. | | 1693575
 |
Mitosis-specific monoclonal antibody MPM-2 inhibits Xenopus oocyte maturation and depletes maturation-promoting activity.
Kuang, J, et al.
Proc. Natl. Acad. Sci. U.S.A., 86: 4982-6 (1989)
1988
Mostrar resumen
MPM-2, a monoclonal antibody specific for cells in mitosis, recognizes a family of proteins that share a common phosphorylated epitope. In this study we have shown that during the maturation of Xenopus laevis oocytes induced by progesterone, phosphorylation of MPM-2 antigens coincided with the appearance of MPF activity. When MPM-2 (0.7-1.4 micrograms per oocyte) was injected into oocytes prior to progesterone stimulation, MPF activity failed to appear and induction of maturation was inhibited as judged by both germinal-vesicle breakdown and white-spot formation. Further, MPM-2 was able to neutralize as well as immunodeplete MPF activity from mitotic HeLa cell and mature oocyte extracts. These results suggest that MPM-2 recognizes either MPF itself or a protein(s) that regulates MPF activity and that the kinase that phosphorylates MPM-2 antigens may be a key component in the regulation of M-phase induction. | | 2662192
 |
Phosphoproteins are components of mitotic microtubule organizing centers.
Vandre, D D, et al.
Proc. Natl. Acad. Sci. U.S.A., 81: 4439-43 (1984)
1983
Mostrar resumen
Protein phosphorylation has been suggested as an important control mechanism for the events leading toward the initiation and completion of mitosis. Using a monoclonal antibody recognizing a class of phosphoproteins abundant in mitotic cells, we demonstrated the localization of a subset of these phosphoproteins to several discrete mitotic structures. Patchy immunofluorescence was present in the interphase nuclei, but a significant increase in nuclear immunofluorescence was apparent at prophase. Subsequent mitotic stages demonstrated that immunoreactive material was particularly apparent at microtubule organizing centers, namely, centrosomes, kinetochores, and midbodies. Intense centrosomal localization occurred at the prophase-prometaphase transition and persisted until the reformation of the nuclear membrane in early G1. The cytoplasm of mitotic cells also contained immunoreactive material in sharp contrast to interphase cells that exhibited no cytoplasmic fluorescent staining. Much of the diffuse immunofluorescent cytoplasmic material was removed by a brief lysis of the cells with 0.15% Triton X-100 prior to fixation. The localization of the remaining immunoreactive material after detergent lysis to mitotic microtubule organizing centers suggests that they contain phosphoprotein structural components important, perhaps, in the mitotic phase-interphase transition. | | 6379644
 |
Monoclonal antibodies to mitotic cells.
Davis, F M, et al.
Proc. Natl. Acad. Sci. U.S.A., 80: 2926-30 (1983)
1982
Mostrar resumen
Certain proteins or activities are present in mitotic cells but not in interphase cells. These proteins may be synthesized or activated, or both, just prior to mitosis and are responsible for the breakdown of the nuclear envelope and the condensation of chromosomes. To learn more about the nature of these proteins, we raised monoclonal antibodies to mitotic cells. Spleen cells from mice immunized with a 0.15 M NaCl extract of synchronized mitotic HeLa cells were fused with SP2/0-Ag14 mouse myeloma cells, and hybrids were selected in medium containing hypoxanthine, methotrexate, thymidine, and glycine. Two different hybridoma clones secreting antibodies reactive with mitotic and meiotic cells from every species tested were isolated. Chromosomes as well as cytoplasm in mitotic cells reacted with the antibodies, as detected by indirect immunofluorescence. The proteins from mitotic cells were separated by electrophoresis in NaDodSO4/polyacrylamide slab gels, transferred to nitrocellulose sheets, and stained immunochemically. The two antibodies, designated MPM-1 and MPM-2, recognize a family of polypeptides with apparent molecular masses of 0.40 to greater than 200 kilodaltons (kDa). Both antibodies reacted strongly with three polypeptide bands of 182 kDa, 118 kDa, and 70 kDa. Only mitotic cells exhibited the protein bands that were recognized by the antibodies. All these bands were found to be phosphoproteins as shown by 32P labeling and autoradiography and their removal by alkaline phosphatase treatment. | | 6574461
 |