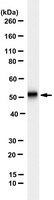
NMDAR mediated translation at the synapse is regulated by MOV10 and FMRP.
Kute PM, Ramakrishna S, Neelagandan N, Chattarji S, Muddashetty RS.
Mol Brain
12(1)
65
2019
Mostrar resumen
Protein synthesis is crucial for maintaining synaptic plasticity and synaptic signalling. Here we have attempted to understand the role of RNA binding proteins, Fragile X Mental Retardation Protein (FMRP) and Moloney Leukemia Virus 10 (MOV10) protein in N-Methyl-D-Aspartate Receptor (NMDAR) mediated translation regulation. We show that FMRP is required for translation downstream of NMDAR stimulation and MOV10 is the key specificity factor in this process. In rat cortical synaptoneurosomes, MOV10 in association with FMRP and Argonaute 2 (AGO2) forms the inhibitory complex on a subset of NMDAR responsive mRNAs. On NMDAR stimulation, MOV10 dissociates from AGO2 and promotes the translation of its target mRNAs. FMRP is required to form MOV10-AGO2 inhibitory complex and to promote translation of MOV10 associated mRNAs. Phosphorylation of FMRP appears to be the potential switch for NMDAR mediated translation and in the absence of FMRP, the distinct translation response to NMDAR stimulation is lost. Thus, FMRP and MOV10 have an important regulatory role in NMDAR mediated translation at the synapse. | 31291981
 |
Polypyrrole/polylactic acid nanofibrous scaffold cotransplanted with bone marrow stromal cells promotes the functional recovery of spinal cord injury in rats.
Raynald, Shu B, Liu XB, Zhou JF, Huang H, Wang JY, Sun XD, Qin C, An YH.
CNS Neurosci Ther
25(9)
951-964
2019
Mostrar resumen
The objective of this study was to analyze the efficacy of polypyrrole/polylactic acid (PPy/PLA) nanofibrous scaffold cotransplanted with bone marrow stromal cells (BMSCs) in promoting the functional recovery in a rat spinal cord injury (SCI). | 31486601
 |
Microfabricated intracortical extracellular matrix-microelectrodes for improving neural interfaces.
Shen W, Das S, Vitale F, Richardson A, Ananthakrishnan A, Struzyna LA, Brown DP, Song N, Ramkumar M, Lucas T, Cullen DK, Litt B, Allen MG.
Microsyst Nanoeng
4
30
2018
Mostrar resumen
Intracortical neural microelectrodes, which can directly interface with local neural microcircuits with high spatial and temporal resolution, are critical for neuroscience research, emerging clinical applications, and brain computer interfaces (BCI). However, clinical applications of these devices remain limited mostly by their inability to mitigate inflammatory reactions and support dense neuronal survival at their interfaces. Herein we report the development of microelectrodes primarily composed of extracellular matrix (ECM) proteins, which act as a bio-compatible and an electrochemical interface between the microelectrodes and physiological solution. These ECM-microelectrodes are batch fabricated using a novel combination of micro-transfer-molding and excimer laser micromachining to exhibit final dimensions comparable to those of commercial silicon-based microelectrodes. These are further integrated with a removable insertion stent which aids in intracortical implantation. Results from electrochemical models and in vivo recordings from the rat's cortex indicate that ECM encapsulations have no significant effect on the electrochemical impedance characteristics of ECM-microelectrodes at neurologically relevant frequencies. ECM-microelectrodes are found to support a dense layer of neuronal somata and neurites on the electrode surface with high neuronal viability and exhibited markedly diminished neuroinflammation and glial scarring in early chronic experiments in rats. | 31057918
 |
Initial tract formation in the mouse brain.
Easter SS Jr, Ross LS, Frankfurter A.
J Neurosci
13(1)
285-99
1992
Mostrar resumen
Mouse embryos from embryonic days 8.5-10.5 (E8.5-E10.5) were fixed and labeled with an antibody to neuron-specific class III beta-tubulin (Moody et al., 1987; Lee et al., 1990a,b) to reveal the first neurons, axons, and tracts in the brain. They were studied in whole-mounts and in light microscopic sections. Some conclusions were checked by labeling tracts in older embryos (E11.5 and E12.5) with the lipophilic dye 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine. The first immunoreactive cells appeared at E8.5, prior to neural tube closure, in the neural plate immediately caudal to the optic vesicle. Cells along the dorsal midline of the mesencephalon issued the first axons, on E9.0; the cells were the mesencephalic nucleus of the trigeminal nerve, and the axons formed its descending tract. The tract reached the level of the trigeminal ganglion by E10.0 but did not enter the ganglion until after E12.5. On E9.5, the number of labeled cells and axons in the alar plate of the presumptive diencephalon and mesencephalon had increased substantially, and many of the rostral ones coursed into the basal plate to enter longitudinal tracts there. Two tracts originated from cells in the basal plate: the tract of the postoptic commissure (from the base of the optic stalk to the level of the cephalic flexure) and the medial longitudinal fasciculus (from the level of the cephalic flexure caudally through the mid and hind-brains). By E10.0, a small mammillotegmental tract paralleled the tract of the postoptic commissure, but immunolabeling was so widespread that discrete tracts were impossible to discern in the presumptive diencephalon and mesencephalon. The more rostral regions remained lightly labeled. In the cerebral vesicle, the presumptive cerebral cortex, the first immunoreactive cells appeared at E10.0; they had multiple processes oriented parallel to the pia, and were identified as the Cajal-Retzius cells. By E10.5, no tracts had formed in the cerebral vesicle. All the tracts formed by E10.0 were superficial, in the subpial lamina. Those that can be identified in the adult brain are very deep structures. These results are compared with previous descriptions of the embryonic brains of amphibians, fish, birds, and other mammals, including humans. | 8423474
 |
Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry.
Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, MacDonald TL, Sundberg RJ, Rebhun LI, Frankfurter A.
Proc Natl Acad Sci U S A
88(11)
4685-9
1991
Mostrar resumen
Class III beta-tubulin, isolated from adult bovine brain, is resolved into at least seven charge variants on isoelectric focusing gels. To identify the posttranslational modifications responsible for this heterogeneity, a mixture of brain tubulins was treated with cyanogen bromide and the C-terminal fragments from the class III beta-tubulin isoforms were then isolated by binding them to the monoclonal antibody TuJ1. Combined use of tandem mass spectrometry and both subtractive and automated Edman degradation chemistry on the isolated peptides indicates that many of the isoforms differ by phosphorylation at Ser-444 plus attachment of one to six glutamic acid molecules to the side chain of the first glutamate residue, Glu-438, in the C-terminal sequence Tyr-Glu-Asp-Asp-Glu-Glu-Glu-Ser-glu-Ala-Gln-Gly-Pro-Lys. | 2052551
 |