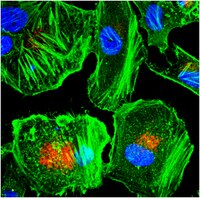
The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein.
Alpy, F; Stoeckel, ME; Dierich, A; Escola, JM; Wendling, C; Chenard, MP; Vanier, MT; Gruenberg, J; Tomasetto, C; Rio, MC
The Journal of biological chemistry
276
4261-9
2001
Mostrar resumen
MLN64 is a transmembrane protein that shares homology with the cholesterol binding domain (START domain) of the steroidogenic acute regulatory protein. The steroidogenic acute regulatory protein is located in the inner membrane of mitochondria, where it facilitates cholesterol import into the mitochondria. Crystallographic analysis showed that the START domain of MLN64 is a cholesterol-binding domain. The present work was undertaken to determine which step of the intracellular cholesterol pathway MLN64 participates in. Using immunocytofluorescence, MLN64 colocalizes with LBPA, a lipid found specifically in late endosomes. Electron microscopy indicates that MLN64 is restricted to the limiting membrane of late endosomes. Microinjection or endocytosis of specific antibodies shows that the START domain of MLN64 is cytoplasmic. Deletion and mutagenesis experiments demonstrate that the amino-terminal part of MLN64 is responsible for its addressing. Although this domain does not contain conventional dileucine- or tyrosine-based targeting signals, we show that a dileucine motif (Leu(66)-Leu(67)) and a tyrosine residue (Tyr(89)) are critical for the targeting or the proper folding of the molecule. Finally, MLN64 colocalizes with cholesterol and Niemann Pick C1 protein in late endosomes. However, complementation assays show that MLN64 is not involved in the Niemann Pick C2 disease which, results in cholesterol lysosomal accumulation. Together, our results show that MLN64 plays a role at the surface of the late endosomes, where it might shuttle cholesterol from the limiting membrane to cytoplasmic acceptor(s). | 11053434
 |
Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport.
Kobayashi, T; Beuchat, MH; Lindsay, M; Frias, S; Palmiter, RD; Sakuraba, H; Parton, RG; Gruenberg, J
Nature cell biology
1
113-8
1998
Mostrar resumen
The fate of free cholesterol released after endocytosis of low-density lipoproteins remains obscure. Here we report that late endosomes have a pivotal role in intracellular cholesterol transport. We find that in the genetic disease Niemann-Pick type C (NPC), and in drug-treated cells that mimic NPC, cholesterol accumulates in late endosomes and sorting of the lysosomal enzyme receptor is impaired. Our results show that the characteristic network of lysobisphosphatidic acid-rich membranes contained within multivesicular late endosomes regulates cholesterol transport, presumably by acting as a collection and distribution device. The results also suggest that similar endosomal defects accompany the anti-phospholipid syndrome and NPC. | 10559883
 |
A lipid associated with the antiphospholipid syndrome regulates endosome structure and function.
Kobayashi, T; Stang, E; Fang, KS; de Moerloose, P; Parton, RG; Gruenberg, J
Nature
392
193-7
1998
Mostrar resumen
Little is known about the structure and function of membrane domains in the vacuolar apparatus of animal cells. A unique feature of late endosomes, which are part of the pathway that leads to lysosomes, is that they contain a complex system of poorly characterized internal membranes in their lumen. These endosomes are therefore known as multivesicular or multilamellar organelles. Some proteins distribute preferentially within these internal membranes, whereas others are exclusively localized to the organelle's limiting membrane. The composition and function of this membrane system are poorly understood. Here we show that these internal membranes contain large amounts of a unique lipid, and thus form specialized domains within endosomes. These specialized domains are involved in sorting the multifunctional receptor for insulin-like growth factor 2 and ligands bearing mannose-6-phosphate, in particular lysosomal enzymes. We also show that this unique lipid is a specific antigen for human antibodies associated with the antiphospholipid syndrome. These antibodies may act intracellularly by altering the protein-sorting functions of endosomes. | 9515966
 |