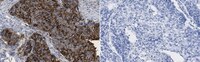
Deficiency of a sulfotransferase for sialic acid-modified glycans mitigates Alzheimer's pathology
Zui Zhang 1 , Yoshiko Takeda-Uchimura 1 , Tahmina Foyez 1 , Shiori Ohtake-Niimi 1 , Narentuya 1 , Hiroyasu Akatsu 2 3 , Kazuchika Nishitsuji 4 , Makoto Michikawa 5 6 , Tony Wyss-Coray 7 , Kenji Kadomatsu 1 , Kenji Uchimura
Proc Natl Acad Sci U S A
114(14)
E2947-E2954
2016
Mostrar resumen
We previously showed that microglial keratan sulfate (KS) was induced in amyotrophic lateral sclerosis. However, the functional roles of the glycan and its synthetic enzyme in neurodegenerative diseases, such as Alzheimer's disease (AD), a progressive disorder, are unclear. In our study, KS modified with sialic acids having a molecular mass of 125-220 kDa and the carbohydrate sulfotransferase GlcNAc6ST1 were up-regulated in the brains of two transgenic mouse models (J20 and Tg2576) and the brains of patients with AD. GlcNAc6ST1-deficient J20 (J20/GlcNAc6ST1-/-) mice demonstrated a complete absence of the microglial sialylated KS. J20/GlcNAc6ST1-/- primary microglia showed an increased level of amyloid-β phagocytosis and were hyperresponsive to interleukin 4, a potent antiinflammatory cytokine. Moreover, J20/GlcNAc6ST1-/- mice manifested reduced cerebral amyloid-β deposition. GlcNAc6ST1-synthesizing sialylated KS thus modulates AD pathology. Inhibition of KS synthesis by targeting GlcNAc6ST1 may therefore be beneficial for controlling AD pathogenesis. | 28320965
 |
Mast cells produce novel shorter forms of perlecan that contain functional endorepellin: a role in angiogenesis and wound healing
Moonsun Jung 1 , Megan S Lord, Bill Cheng, J Guy Lyons, Hatem Alkhouri, J Margaret Hughes, Simon J McCarthy, Renato V Iozzo, John M Whitelock
J Biol Chem
288(5)
3289-304
2013
Mostrar resumen
Mast cells are derived from hematopoietic progenitors that are known to migrate to and reside within connective and mucosal tissues, where they differentiate and respond to various stimuli by releasing pro-inflammatory mediators, including histamine, growth factors, and proteases. This study demonstrated that primary human mast cells as well as the rat and human mast cell lines, RBL-2H3 and HMC-1, produce the heparan sulfate proteoglycan, perlecan, with a molecular mass of 640 kDa as well as smaller molecular mass species of 300 and 130 kDa. Utilizing domain-specific antibodies coupled with N-terminal sequencing, it was confirmed that both forms contained the C-terminal module of the protein core known as endorepellin, which were generated by mast cell-derived proteases. Domain-specific RT-PCR experiments demonstrated that transcripts corresponding to domains I and V, including endorepellin, were present; however, mRNA transcripts corresponding to regions of domain III were not present, suggesting that these cells were capable of producing spliced forms of the protein core. Fractions from mast cell cultures that were enriched for these fragments were shown to bind endothelial cells via the α(2)β(1) integrin and stimulate the migration of cells in "scratch assays," both activities of which were inhibited by incubation with either anti-endorepellin or anti-perlecan antibodies. This study shows for the first time that mast cells secrete and process the extracellular proteoglycan perlecan into fragments containing the endorepellin C-terminal region that regulate angiogenesis and matrix turnover, which are both key events in wound healing. | 23235151
 |
Effect of depletion of interstitial hyaluronan on hydraulic conductance in rabbit knee synovium
P J Coleman 1 , D Scott, A Abiona, D E Ashhurst, R M Mason, J R Levick
J Physiol
509 ( Pt 3)(Pt 3)
695-710
1998
Mostrar resumen
1. The hydraulic resistance of the synovial lining to fluid outflow from a joint cavity (Qs) is important for the retention of intra-articular lubricant. The resistance has been attributed in part to extracellular glycosaminoglycans, including hyaluronan and chondroitin sulphates. Increased permeability in joints infused with testicular hyaluronidase, which digests both chondroitin sulphates and hyaluronan, supports this view. In this study the importance of interstitial hyaluronan per se was assessed using leech and Streptomyces hyaluronidases, which degrade only hyaluronan. 2. Ringer solution was infused into the knee joint cavity of anaesthetized rabbits for 30 min, with or without hyaluronidase, after which intra-articular pressure (Pj) was raised and the relation between pressure and outflow determined. 3. Treatment with Streptomyces, leech or testicular hyaluronidases increased the fluid escape rates by similar factors, namely 4- to 6-fold. After Streptomyces hyaluronidase treatment the slope d 8d s/dPj, which at low pressures represents synovial hydraulic conductance, increased from a control of 0.90 +/- 0.20 microl min-1 cmH2O-1 (mean +/- s.e.m. , n = 6) to 4.52 +/- 0.70 microl min-1 cmH2O-1. The slope d 8d s/dPj increased to a similar level after testicular hyaluronidase, namely to 4.14 +/- 1.06 microl min-1 cmH2O-1 (control, 0.54 +/- 0.24 microl min-1 cmH2O-1). Streptomyces and leech hyaluronidases were as effective as testicular hyaluronidase (no statistically significant differences) despite differences in substrate specificity. 4. It was shown using histochemical and immunohistochemical techniques that hyaluronan was removed from the synovium by leech, Streptomyces and testicular hyaluronidases. The binding of antibodies 2-B-6 and 3-B-3 showed that the core proteins of the chondroitin sulphate proteoglycans remained intact after treatment with hyaluronidases, and the binding of 5-D-4 showed that keratan sulphate was unaffected. An azocasein digestion assay confirmed that the hyaluronidase preparations had no significant proteolytic activity. 5. The effect of the hyaluronidases was four times greater than predicted from the low concentration of interstitial hyaluronan and its resistivity. Factors that might amplify the effect of hyaluronan depletion include the matrix-organizing role of hyaluronan, and/or non-uniformity of hyaluronan distribution. It is concluded that interstitial hyaluronan makes a major contribution to synovial hydraulic resistance, but the mechanisms are as yet poorly understood. | 9596792
 |
Changes in glycosaminoglycan epitope levels in knee joint fluid following injury
P K Hazell 1 , C Dent, J A Fairclough, M T Bayliss, T E Hardingham
Arthritis Rheum
38(7)
953-9
1994
Mostrar resumen
Objective: To measure the levels of epitope on the chondroitin sulfate (CS) and keratan sulfate (KS) chains of proteoglycan fragments in synovial fluids from injured and contralateral uninjured knees of patients with traumatic cruciate ligament and/or meniscus damage. <br />Methods: Enzyme-linked immunosorbent assays were used to determine the levels of monoclonal antibody epitopes 3-B-3 and 7-D-4 (CS), and 5-D-4 (KS), in paired joint fluids from the injured and uninjured knees of trauma patients. <br />Results: Levels of the CS epitopes were increased in the trauma joint fluids from most patients, with higher levels of 3-B-3 epitope in 12 of the 16 patients, but the difference did not achieve significance; however, 7-D-4 levels were higher in 15 patients, and the difference was highly significant (P = 0.0005). In contrast, the KS epitope detected by 5-D-4 was decreased in 13 of 15 patients, and the difference was significant (P = 0.0074). <br />Conclusion: The increased level of 7-D-4 epitope on proteoglycans in joint fluid from injured knees may reflect the response of the articular cartilage to acute trauma resulting in altered expression of specific CS epitopes on cartilage proteoglycans. The fall in KS epitope levels may reflect the synthesis of proteoglycans that have lower KS content. | 7541993
 |
Menstrual-cycle-dependent expression of keratan sulphate in human endometrium
M E Hoadley 1 , M W Seif, J D Aplin
Biochem J
266(3)
757-63
1990
Mostrar resumen
Immunochemical methods have been used to detect and characterize two classes of polypeptide-associated keratan sulphate (KS) in epithelial secretions from human endometrium. Monoclonal antibody D9B1 binds to a hormonally regulated sialylated epitope associated with KS in a high relative molecular mass (250,000-350,000) component that bands as a doublet in SDS/PAGE. These KS chain(s) are sensitive to keratanase, endo-beta-galactosidase and N-glycanase. A second, more highly sulphated, type of KS is also present, that is resistant to all three enzymes. This can be detected using monoclonal antibody 5D4. It is present throughout the menstrual cycle and is associated principally with a component of Mr 140,000. Thus secretory KS contributes to the environment of the implanting embryo, may be used as a molecular index of endometrial function and could be important in the establishment of pregnancy. | 1691631
 |
Immunological methods for the detection and determination of connective tissue proteoglycans
B Caterson, J R Baker, J E Christner, J R Couchman
J Invest Dermatiol
79 Suppl 1
45s-50s
1981
Mostrar resumen
In this paper we report the use of immunological methods for specifically detecting and determining proteoglycan in cartilage and other connective tissues. Antibodies (polyclonal and monoclonal) have been raised against specific components of cartilage proteoglycan aggregates (i.e., proteoglycan monomer and link protein). Radioimmunoassay procedures and immunohistochemical procedures have been developed and used to demonstrate the occurrence of cartilage-like proteoglycan and link protein in bovine aorta. Similarly, immunofluorescent studies have been used to analyze proteoglycan distribution in skin. Using antibodies specific for chondroitin-4-sulfated proteoglycan, their presence was demonstrated in dermal connective tissue and connective tissue surrounding nerve and muscle sheaths. However, chondroitin-4-sulfated proteoglycan was completely absent in the epidermis of skin and areas surrounding invaginating hair follicles. These immunological procedures are currently being used to complement conventional biochemical analyses of proteoglycans found in different connective tissue matrices. | 6806399
 |