Specific in situ detection of murine indoleamine 2, 3-dioxygenase.
Thomas, S; DuHadaway, J; Prendergast, GC; Laury-Kleintop, L
Journal of cellular biochemistry
115
391-6
2014
Mostrar resumen
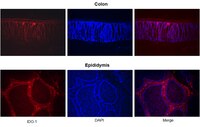
Indoleamine 2,3-dioxygenase-1 (IDO1) catabolizes the essential amino acid tryptophan, acting as a modifier of inflammation and immune tolerance. Recent work has implicated IDO1 in many human diseases, including in cancer, chronic infection, autoimmune disorders, and neurodegenerative disease, stimulating a major surge in preclinical and clinical studies of its pathogenic functions. In the mouse, IDO1 is expressed widely but in situ detection of the enzyme in murine tissues has been unreliable due to the lack of specific antibodies that do not also react with tissues from animals that are genetically deficient in IDO1. Such probes are crucial to establish cellular mechanisms since IDO1 appears to act in different cell types depending on disease context, but reliable probes have been elusive in the field. In this report, we address this issue with the development of IDO1 monoclonal antibody 4B7 which specifically recognizes the murine enzyme in tissue sections, offering a reliable tool for immunohistology in preclinical disease models. | 24123235
 |