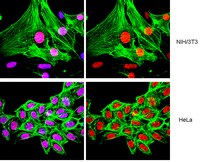
hnRNP K coordinates transcriptional silencing by SETDB1 in embryonic stem cells.
Thompson, PJ; Dulberg, V; Moon, KM; Foster, LJ; Chen, C; Karimi, MM; Lorincz, MC
PLoS genetics
11
e1004933
2015
Mostrar resumen
Retrotransposition of endogenous retroviruses (ERVs) poses a substantial threat to genome stability. Transcriptional silencing of a subset of these parasitic elements in early mouse embryonic and germ cell development is dependent upon the lysine methyltransferase SETDB1, which deposits H3K9 trimethylation (H3K9me3) and the co-repressor KAP1, which binds SETDB1 when SUMOylated. Here we identified the transcription co-factor hnRNP K as a novel binding partner of the SETDB1/KAP1 complex in mouse embryonic stem cells (mESCs) and show that hnRNP K is required for ERV silencing. RNAi-mediated knockdown of hnRNP K led to depletion of H3K9me3 at ERVs, concomitant with de-repression of proviral reporter constructs and specific ERV subfamilies, as well as a cohort of germline-specific genes directly targeted by SETDB1. While hnRNP K recruitment to ERVs is dependent upon KAP1, SETDB1 binding at these elements requires hnRNP K. Furthermore, an intact SUMO conjugation pathway is necessary for SETDB1 recruitment to proviral chromatin and depletion of hnRNP K resulted in reduced SUMOylation at ERVs. Taken together, these findings reveal a novel regulatory hierarchy governing SETDB1 recruitment and in turn, transcriptional silencing in mESCs. | Western Blotting | | 25611934
 |
Functional complementation of sir2Δ yeast mutation by the human orthologous gene SIRT1.
Gaglio, D; D'Alfonso, A; Camilloni, G
PloS one
8
e83114
2013
Mostrar resumen
Sirtuins, class III histone deacetylases, are proteins homologous to the yeast protein Sir2p. Mammalian Sirt1 has been shown to be involved in energy metabolism, brain functions, inflammation and aging through its deacetylase activity, acting on both histone and non-histone substrates. In order to verify whether Sirt1 can replace Sir2p in the yeast cells, we expressed the full-length human Sirt1 protein in S.cerevisiae sir2Δ mutant strain. The structure of chromatin is basically maintained from yeast to human. Thus, yeast chromatin is a favourable environment to evaluate, inhibit or activate an ectopic histone deacetylase activity in an in vivo substrate. Mutant sir2Δ shows a series of different phenotypes, all dependent on the deacetylase activity of Sir2p. We analyzed the three silent loci where normally Sir2p acts: ribosomal DNA, telomeres and the mating type loci. Moreover, we verified extrachromosomal ribosomal DNA circles production and histone hyperacetylation levels, typical marks of sir2Δ strains. By strong SIRT1 overexpression in sir2Δ cells, we found that specific molecular phenotypes of the mutant revert almost to a wild-type condition. In particular, transcriptional silencing at rDNA was restored, extrachromosomal rDNA circles formation was repressed and histone acetylation at H3K9 and H4K16 decreased. The complementation at the other studied loci: HM loci, telomere and sub-telomere does not occur. Overall, our observations indicate that: i) SIRT1 gene is able to complement different molecular phenotypes of the sir2Δ mutant at rDNA ii) the in vivo screening of Sirt1 activity is possible in yeast. | | | 24349441
 |
A selective HDAC 1/2 inhibitor modulates chromatin and gene expression in brain and alters mouse behavior in two mood-related tests.
Schroeder, FA; Lewis, MC; Fass, DM; Wagner, FF; Zhang, YL; Hennig, KM; Gale, J; Zhao, WN; Reis, S; Barker, DD; Berry-Scott, E; Kim, SW; Clore, EL; Hooker, JM; Holson, EB; Haggarty, SJ; Petryshen, TL
PloS one
8
e71323
2013
Mostrar resumen
Psychiatric diseases, including schizophrenia, bipolar disorder and major depression, are projected to lead global disease burden within the next decade. Pharmacotherapy, the primary--albeit often ineffective--treatment method, has remained largely unchanged over the past 50 years, highlighting the need for novel target discovery and improved mechanism-based treatments. Here, we examined in wild type mice the impact of chronic, systemic treatment with Compound 60 (Cpd-60), a slow-binding, benzamide-based inhibitor of the class I histone deacetylase (HDAC) family members, HDAC1 and HDAC2, in mood-related behavioral assays responsive to clinically effective drugs. Cpd-60 treatment for one week was associated with attenuated locomotor activity following acute amphetamine challenge. Further, treated mice demonstrated decreased immobility in the forced swim test. These changes are consistent with established effects of clinical mood stabilizers and antidepressants, respectively. Whole-genome expression profiling of specific brain regions (prefrontal cortex, nucleus accumbens, hippocampus) from mice treated with Cpd-60 identified gene expression changes, including a small subset of transcripts that significantly overlapped those previously reported in lithium-treated mice. HDAC inhibition in brain was confirmed by increased histone acetylation both globally and, using chromatin immunoprecipitation, at the promoter regions of upregulated transcripts, a finding consistent with in vivo engagement of HDAC targets. In contrast, treatment with suberoylanilide hydroxamic acid (SAHA), a non-selective fast-binding, hydroxamic acid HDAC 1/2/3/6 inhibitor, was sufficient to increase histone acetylation in brain, but did not alter mood-related behaviors and had dissimilar transcriptional regulatory effects compared to Cpd-60. These results provide evidence that selective inhibition of HDAC1 and HDAC2 in brain may provide an epigenetic-based target for developing improved treatments for mood disorders and other brain disorders with altered chromatin-mediated neuroplasticity. | Western Blotting | Mouse | 23967191
 |
DNMT1 is regulated by ATP-citrate lyase and maintains methylation patterns during adipocyte differentiation.
Londoño Gentile, T; Lu, C; Lodato, PM; Tse, S; Olejniczak, SH; Witze, ES; Thompson, CB; Wellen, KE
Molecular and cellular biology
33
3864-78
2013
Mostrar resumen
During adipocyte differentiation, significant epigenomic changes occur in association with the implementation of the adipogenic program. We have previously shown that histone acetylation increases during differentiation in a manner dependent on acetyl coenzyme A (acetyl-CoA) production by the enzyme ATP-citrate lyase (ACL). Whether ACL regulates nuclear targets in addition to histones during differentiation is not clear. In this study, we report that DNA methyltransferase 1 (DNMT1) levels in adipocytes are controlled in part by ACL and that silencing of DNMT1 can accelerate adipocyte differentiation. DNMT1 gene expression is induced early in 3T3-L1 adipocyte differentiation during mitotic clonal expansion and is critical for maintenance of DNA and histone H3K9 methylation patterns during this period. In the absence of DNMT1, adipocyte-specific gene expression and lipid accumulation occur precociously. Later in differentiation, DNMT1 levels decline in an ACL-dependent manner. ACL-mediated suppression of DNMT1 occurs at least in part by promoting expression of microRNA 148a (miR-148a), which represses DNMT1. Ectopic expression of miR-148a accelerates differentiation under standard conditions and can partially rescue a hypermethylation-mediated differentiation block. The data suggest a role for DNMT1 in modulating the timing of differentiation and describe a novel ACL-miR-148a-dependent mechanism for regulating DNMT1 during adipogenesis. | Western Blotting | | 23897429
 |
Specific acetylation of p53 by HDAC inhibition prevents DNA damage-induced apoptosis in neurons.
Brochier, C; Dennis, G; Rivieccio, MA; McLaughlin, K; Coppola, G; Ratan, RR; Langley, B
The Journal of neuroscience : the official journal of the Society for Neuroscience
33
8621-32
2013
Mostrar resumen
Histone deacetylase (HDAC) inhibitors have been used to promote neuronal survival and ameliorate neurological dysfunction in a host of neurodegenerative disease models. The precise molecular mechanisms whereby HDAC inhibitors prevent neuronal death are currently the focus of intensive research. Here we demonstrate that HDAC inhibition prevents DNA damage-induced neurodegeneration by modifying the acetylation pattern of the tumor suppressor p53, which decreases its DNA-binding and transcriptional activation of target genes. Specifically, we identify that acetylation at K382 and K381 prevents p53 from associating with the pro-apoptotic PUMA gene promoter, activating transcription, and inducing apoptosis in mouse primary cortical neurons. Paradoxically, acetylation of p53 at the same lysines in various cancer cell lines leads to the induction of PUMA expression and death. Together, our data provide a molecular understanding of the specific outcomes of HDAC inhibition and suggest that strategies aimed at enhancing p53 acetylation at K381 and K382 might be therapeutically viable for capturing the beneficial effects in the CNS, without compromising tumor suppression. | | | 23678107
 |
Replication-uncoupled histone deposition during adenovirus DNA replication.
Komatsu, T; Nagata, K
J Virol
86
6701-11
2011
Mostrar resumen
In infected cells, the chromatin structure of the adenovirus genome DNA plays critical roles in its genome functions. Previously, we reported that in early phases of infection, incoming viral DNA is associated with both viral core protein VII and cellular histones. Here we show that in late phases of infection, newly synthesized viral DNA is also associated with histones. We also found that the knockdown of CAF-1, a histone chaperone that functions in the replication-coupled deposition of histones, does not affect the level of histone H3 bound on viral chromatin, although CAF-1 is accumulated at viral DNA replication foci together with PCNA. Chromatin immunoprecipitation assays using epitope-tagged histone H3 demonstrated that histone variant H3.3, which is deposited onto the cellular genome in a replication-independent manner, is selectively associated with both incoming and newly synthesized viral DNAs. Microscopic analyses indicated that histones but not USF1, a transcription factor that regulates viral late gene expression, are excluded from viral DNA replication foci and that this is achieved by the oligomerization of the DNA binding protein (DBP). Taken together, these results suggest that histone deposition onto newly synthesized viral DNA is most likely uncoupled with viral DNA replication, and a possible role of DBP oligomerization in this replication-uncoupled histone deposition is discussed. | | | 22496236
 |
Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps.
Papayannopoulos, V; Metzler, KD; Hakkim, A; Zychlinsky, A
The Journal of cell biology
191
677-91
2009
Mostrar resumen
Neutrophils release decondensed chromatin termed neutrophil extracellular traps (NETs) to trap and kill pathogens extracellularly. Reactive oxygen species are required to initiate NET formation but the downstream molecular mechanism is unknown. We show that upon activation, neutrophil elastase (NE) escapes from azurophilic granules and translocates to the nucleus, where it partially degrades specific histones, promoting chromatin decondensation. Subsequently, myeloperoxidase synergizes with NE in driving chromatin decondensation independent of its enzymatic activity. Accordingly, NE knockout mice do not form NETs in a pulmonary model of Klebsiella pneumoniae infection, which suggests that this defect may contribute to the immune deficiency of these mice. This mechanism provides for a novel function for serine proteases and highly charged granular proteins in the regulation of chromatin density, and reveals that the oxidative burst induces a selective release of granular proteins into the cytoplasm through an unknown mechanism. | Western Blotting | | 20974816
 |