Cellular plasticity induced by anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor encephalitis antibodies.
Peng, X; Hughes, EG; Moscato, EH; Parsons, TD; Dalmau, J; Balice-Gordon, RJ
Annals of neurology
77
381-98
2015
Mostrar resumen
Autoimmune-mediated anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) encephalitis is a severe but treatment-responsive disorder with prominent short-term memory loss and seizures. The mechanisms by which patient antibodies affect synapses and neurons leading to symptoms are poorly understood.The effects of patient antibodies on cultures of live rat hippocampal neurons were determined with immunostaining, Western blot, and electrophysiological analyses.We show that patient antibodies cause a selective decrease in the total surface amount and synaptic localization of GluA1- and GluA2-containing AMPARs, regardless of receptor subunit binding specificity, through increased internalization and degradation of surface AMPAR clusters. In contrast, patient antibodies do not alter the density of excitatory synapses, N-methyl-D-aspartate receptor (NMDAR) clusters, or cell viability. Commercially available AMPAR antibodies directed against extracellular epitopes do not result in a loss of surface and synaptic receptor clusters, suggesting specific effects of patient antibodies. Whole-cell patch clamp recordings of spontaneous miniature postsynaptic currents show that patient antibodies decrease AMPAR-mediated currents, but not NMDAR-mediated currents. Interestingly, several functional properties of neurons are also altered: inhibitory synaptic currents and vesicular γ-aminobutyric acid transporter (vGAT) staining intensity decrease, whereas the intrinsic excitability of neurons and short-interval firing increase.These results establish that antibodies from patients with anti-AMPAR encephalitis selectively eliminate surface and synaptic AMPARs, resulting in a homeostatic decrease in inhibitory synaptic transmission and increased intrinsic excitability, which may contribute to the memory deficits and epilepsy that are prominent in patients with this disorder. | | | 25369168
 |
Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis.
Dong, Z; Han, H; Li, H; Bai, Y; Wang, W; Tu, M; Peng, Y; Zhou, L; He, W; Wu, X; Tan, T; Liu, M; Wu, X; Zhou, W; Jin, W; Zhang, S; Sacktor, TC; Li, T; Song, W; Wang, YT
The Journal of clinical investigation
125
234-47
2015
Mostrar resumen
Long-term potentiation (LTP) of synaptic strength between hippocampal neurons is associated with learning and memory, and LTP dysfunction is thought to underlie memory loss. LTP can be temporally and mechanistically classified into decaying (early-phase) LTP and nondecaying (late-phase) LTP. While the nondecaying nature of LTP is thought to depend on protein synthesis and contribute to memory maintenance, little is known about the mechanisms and roles of decaying LTP. Here, we demonstrated that inhibiting endocytosis of postsynaptic α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptors (AMPARs) prevents LTP decay, thereby converting it into nondecaying LTP. Conversely, restoration of AMPAR endocytosis by inhibiting protein kinase Mζ (PKMζ) converted nondecaying LTP into decaying LTP. Similarly, inhibition of AMPAR endocytosis prolonged memory retention in normal animals and reduced memory loss in a murine model of Alzheimer's disease. These results strongly suggest that an active process that involves AMPAR endocytosis mediates the decay of LTP and that inhibition of this process can prolong the longevity of LTP as well as memory under both physiological and pathological conditions. | | | 25437879
 |
The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition.
Mignogna, ML; Giannandrea, M; Gurgone, A; Fanelli, F; Raimondi, F; Mapelli, L; Bassani, S; Fang, H; Van Anken, E; Alessio, M; Passafaro, M; Gatti, S; Esteban, JA; Huganir, R; D'Adamo, P
Nature communications
6
6504
2015
Mostrar resumen
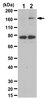
RAB39B is a member of the RAB family of small GTPases that controls intracellular vesicular trafficking in a compartment-specific manner. Mutations in the RAB39B gene cause intellectual disability comorbid with autism spectrum disorder and epilepsy, but the impact of RAB39B loss of function on synaptic activity is largely unexplained. Here we show that protein interacting with C-kinase 1 (PICK1) is a downstream effector of GTP-bound RAB39B and that RAB39B-PICK1 controls trafficking from the endoplasmic reticulum to the Golgi and, hence, surface expression of GluA2, a subunit of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs). The role of AMPARs in synaptic transmission varies depending on the combination of subunits (GluA1, GluA2 and GluA3) they incorporate. RAB39B downregulation in mouse hippocampal neurons skews AMPAR composition towards non GluA2-containing Ca(2+)-permeable forms and thereby alters synaptic activity, specifically in hippocampal neurons. We posit that the resulting alteration in synaptic function underlies cognitive dysfunction in RAB39B-related disorders. | Western Blotting | | 25784538
 |
Diversity of glutamatergic synaptic strength in lateral prefrontal versus primary visual cortices in the rhesus monkey.
Medalla, M; Luebke, JI
The Journal of neuroscience : the official journal of the Society for Neuroscience
35
112-27
2015
Mostrar resumen
Understanding commonalities and differences in glutamatergic synaptic signaling is essential for understanding cortical functional diversity, especially in the highly complex primate brain. Previously, we have shown that spontaneous EPSCs differed markedly in layer 3 pyramidal neurons of two specialized cortical areas in the rhesus monkey, the high-order lateral prefrontal cortex (LPFC) and the primary visual cortex (V1). Here, we used patch-clamp recordings and confocal and electron microscopy to determine whether these distinct synaptic responses are due to differences in firing rates of presynaptic neurons and/or in the features of presynaptic or postsynaptic entities. As with spontaneous EPSCs, TTX-insensitive (action potential-independent) miniature EPSCs exhibited significantly higher frequency, greater amplitude, and slower kinetics in LPFC compared with V1 neurons. Consistent with these physiological differences, LPFC neurons possessed higher densities of spines, and the mean width of large spines was greater compared with those on V1 neurons. Axospinous synapses in layers 2-3 of LPFC had larger postsynaptic density surface areas and a higher proportion of large perforated synapses compared with V1. Axonal boutons in LPFC were also larger in volume and contained ∼ 1.6× more vesicles than did those in V1. Further, LPFC had a higher density of AMPA GluR2 receptor labeling than V1. The properties of spines and synaptic currents of individual layer 3 pyramidal neurons measured here were significantly correlated, consistent with the idea that significantly more frequent and larger synaptic currents are likely due to more numerous, larger, and more powerful synapses in LPFC compared with V1. | | | 25568107
 |
Protein tyrosine phosphatase receptor type R is required for Purkinje cell responsiveness in cerebellar long-term depression.
Erkens, M; Tanaka-Yamamoto, K; Cheron, G; Márquez-Ruiz, J; Prigogine, C; Schepens, JT; Nadif Kasri, N; Augustine, GJ; Hendriks, WJ
Molecular brain
8
1
2015
Mostrar resumen
Regulation of synaptic connectivity, including long-term depression (LTD), allows proper tuning of cellular signalling processes within brain circuitry. In the cerebellum, a key centre for motor coordination, a positive feedback loop that includes mitogen-activated protein kinases (MAPKs) is required for proper temporal control of LTD at cerebellar Purkinje cell synapses. Here we report that the tyrosine-specific MAPK-phosphatase PTPRR plays a role in coordinating the activity of this regulatory loop.LTD in the cerebellum of Ptprr (-/-) mice is strongly impeded, in vitro and in vivo. Comparison of basal phospho-MAPK levels between wild-type and PTPRR deficient cerebellar slices revealed increased levels in mutants. This high basal phospho-MAPK level attenuated further increases in phospho-MAPK during chemical induction of LTD, essentially disrupting the positive feedback loop and preventing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) phosphorylation and endocytosis.Our findings indicate an important role for PTPRR in maintaining low basal MAPK activity in Purkinje cells. This creates an optimal 'window' to boost MAPK activity following signals that induce LTD, which can then propagate through feed-forward signals to cause AMPAR internalization and LTD. | | | 25571783
 |
Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer's disease.
Zhang, J; Hu, M; Teng, Z; Tang, YP; Chen, C
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
14919-33
2014
Mostrar resumen
Abnormal accumulation of β-amyloid (Aβ) is the major neuropathological hallmark of Alzheimer's disease (AD). However, the mechanisms underlying aberrant Aβ formation in AD remain unclear. We showed previously that inhibition of monoacylglycerol lipase (MAGL), the primary enzyme that metabolizes the endocannabinoid 2-arachidonoylglycerol (2-AG) in the brain, robustly reduces Aβ by inhibiting β-site amyloid precursor protein cleaving enzyme 1 (BACE1), a key enzyme responsible for Aβ formation. However, the molecular mechanisms responsible for suppression of BACE1 by inhibition of 2-AG metabolism are largely unknown. We demonstrate here that expression of the noncoding small RNA miR-188-3p that targets BACE1 was significantly downregulated both in the brains of AD humans and APP transgenic (TG) mice, a mouse model of AD. The downregulated miR-188-3p expression was restored by MAGL inhibition. Overexpression of miR-188-3p in the hippocampus reduced BACE1, Aβ, and neuroinflammation and prevented deteriorations in hippocampal basal synaptic transmission, long-term potentiation, spatial learning, and memory in TG mice. 2-AG-induced suppression of BACE1 was prevented by miR-188-3p loss of function. Moreover, miR-188-3p expression was upregulated by 2-AG or peroxisome proliferator-activated receptor-γ (PPARγ) agonists and suppressed by PPARγ antagonism or NF-κB activation. Reducing Aβ and neuroinflammation by MAGL inhibition was occluded by PPARγ antagonism. In addition, BACE1 suppression by 2-AG and PPARγ activation was eliminated by knockdown of NF-κB. Our study provides a novel molecular mechanism underlying improved synaptic and cognitive function in TG mice by 2-AG signaling, which upregulates miR-188-3p expression through PPARγ and NF-κB signaling pathway, resulting in suppressions of BACE1 expression and Aβ formation. | | | 25378159
 |
Extracellular ATP hydrolysis inhibits synaptic transmission by increasing ph buffering in the synaptic cleft.
Vroman, R; Klaassen, LJ; Howlett, MH; Cenedese, V; Klooster, J; Sjoerdsma, T; Kamermans, M
PLoS biology
12
e1001864
2014
Mostrar resumen
Neuronal computations strongly depend on inhibitory interactions. One such example occurs at the first retinal synapse, where horizontal cells inhibit photoreceptors. This interaction generates the center/surround organization of bipolar cell receptive fields and is crucial for contrast enhancement. Despite its essential role in vision, the underlying synaptic mechanism has puzzled the neuroscience community for decades. Two competing hypotheses are currently considered: an ephaptic and a proton-mediated mechanism. Here we show that horizontal cells feed back to photoreceptors via an unexpected synthesis of the two. The first one is a very fast ephaptic mechanism that has no synaptic delay, making it one of the fastest inhibitory synapses known. The second one is a relatively slow (τ≈200 ms), highly intriguing mechanism. It depends on ATP release via Pannexin 1 channels located on horizontal cell dendrites invaginating the cone synaptic terminal. The ecto-ATPase NTPDase1 hydrolyses extracellular ATP to AMP, phosphate groups, and protons. The phosphate groups and protons form a pH buffer with a pKa of 7.2, which keeps the pH in the synaptic cleft relatively acidic. This inhibits the cone Ca²⁺ channels and consequently reduces the glutamate release by the cones. When horizontal cells hyperpolarize, the pannexin 1 channels decrease their conductance, the ATP release decreases, and the formation of the pH buffer reduces. The resulting alkalization in the synaptic cleft consequently increases cone glutamate release. Surprisingly, the hydrolysis of ATP instead of ATP itself mediates the synaptic modulation. Our results not only solve longstanding issues regarding horizontal cell to photoreceptor feedback, they also demonstrate a new form of synaptic modulation. Because pannexin 1 channels and ecto-ATPases are strongly expressed in the nervous system and pannexin 1 function is implicated in synaptic plasticity, we anticipate that this novel form of synaptic modulation may be a widespread phenomenon. | | | 24844296
 |
In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression.
Liu, Z; Fang, J; Dearman, J; Zhang, L; Zuo, J
PloS one
9
e89377
2014
Mostrar resumen
Regeneration of auditory hair cells (HCs) is a promising approach to restore hearing. Recent studies have demonstrated that induced pluripotent stem cells/embryonic stem cells or supporting cells (SCs) adjacent to HCs can be converted to adopt the HC fate. However, little is known about whether new HCs are characteristic of outer or inner HCs. Here, we showed in vivo conversion of 2 subtypes of SCs, inner border cells (IBs) and inner phalangeal cells (IPhs), to the inner HC (IHC) fate. This was achieved by ectopically activating Atoh1, a transcription factor necessary for HC fate, in IBs/IPhs at birth. Atoh1+ IBs/IPhs first turned on Pou4f3, another HC transcription factor, before expressing 8 HC markers. The conversion rate gradually increased from ∼ 2.4% at 1 week of age to ∼ 17.8% in adult. Interestingly, new HCs exhibited IHC characteristics such as straight line-shaped stereociliary bundles, expression of Fgf8 and otoferlin, and presence of larger outward currents than those of outer HCs. However, new HCs lacked the terminal differentiation IHC marker vGlut3, exhibited reduced density of presynaptic Cbtp2 puncta that had little postsynaptic GluR2 specialization, and displayed immature IHC outward currents. Our results demonstrate that the conversion rate of IBs/IPhs in vivo by Atoh1 ectopic expression into the IHC fate was higher and faster and the conversion was more complete than that of the 2 other SC subtypes underneath the outer HCs; however, these new IHCs are arrested before terminal differentiation. Thus, IBs/IPhs are good candidates to regenerate IHCs in vivo. | | | 24586731
 |
The MK2/3 cascade regulates AMPAR trafficking and cognitive flexibility.
Eales, KL; Palygin, O; O'Loughlin, T; Rasooli-Nejad, S; Gaestel, M; Müller, J; Collins, DR; Pankratov, Y; Corrêa, SA
Nature communications
5
4701
2014
Mostrar resumen
The interplay between long-term potentiation and long-term depression (LTD) is thought to be involved in learning and memory formation. One form of LTD expressed in the hippocampus is initiated by the activation of the group 1 metabotropic glutamate receptors (mGluRs). Importantly, mGluRs have been shown to be critical for acquisition of new memories and for reversal learning, processes that are thought to be crucial for cognitive flexibility. Here we provide evidence that MAPK-activated protein kinases 2 and 3 (MK2/3) regulate neuronal spine morphology, synaptic transmission and plasticity. Furthermore, mGluR-LTD is impaired in the hippocampus of MK2/3 double knockout (DKO) mice, an observation that is mirrored by deficits in endocytosis of GluA1 subunits. Consistent with compromised mGluR-LTD, MK2/3 DKO mice have distinctive deficits in hippocampal-dependent spatial reversal learning. These novel findings demonstrate that the MK2/3 cascade plays a strategic role in controlling synaptic plasticity and cognition. | Western Blotting | | 25134715
 |
In vitro ischemia triggers a transcriptional response to down-regulate synaptic proteins in hippocampal neurons.
Fernandes, J; Vieira, M; Carreto, L; Santos, MA; Duarte, CB; Carvalho, AL; Santos, AE
PloS one
9
e99958
2014
Mostrar resumen
Transient global cerebral ischemia induces profound changes in the transcriptome of brain cells, which is partially associated with the induction or repression of genes that influence the ischemic response. However, the mechanisms responsible for the selective vulnerability of hippocampal neurons to global ischemia remain to be clarified. To identify molecular changes elicited by ischemic insults, we subjected hippocampal primary cultures to oxygen-glucose deprivation (OGD), an in vitro model for global ischemia that resulted in delayed neuronal death with an excitotoxic component. To investigate changes in the transcriptome of hippocampal neurons submitted to OGD, total RNA was extracted at early (7 h) and delayed (24 h) time points after OGD and used in a whole-genome RNA microarray. We observed that at 7 h after OGD there was a general repression of genes, whereas at 24 h there was a general induction of gene expression. Genes related with functions such as transcription and RNA biosynthesis were highly regulated at both periods of incubation after OGD, confirming that the response to ischemia is a dynamic and coordinated process. Our analysis showed that genes for synaptic proteins, such as those encoding for PICK1, GRIP1, TARPγ3, calsyntenin-2/3, SAPAP2 and SNAP-25, were down-regulated after OGD. Additionally, OGD decreased the mRNA and protein expression levels of the GluA1 AMPA receptor subunit as well as the GluN2A and GluN2B subunits of NMDA receptors, but increased the mRNA expression of the GluN3A subunit, thus altering the composition of ionotropic glutamate receptors in hippocampal neurons. Together, our results present the expression profile elicited by in vitro ischemia in hippocampal neurons, and indicate that OGD activates a transcriptional program leading to down-regulation in the expression of genes coding for synaptic proteins, suggesting that the synaptic proteome may change after ischemia. | Western Blotting | | 24960035
 |