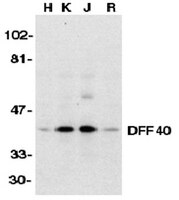
Chromatin collapse during caspase-dependent apoptotic cell death requires DNA fragmentation factor, 40-kDa subunit-/caspase-activated deoxyribonuclease-mediated 3'-OH single-strand DNA breaks.
Iglesias-Guimarais, V; Gil-Guiñon, E; Sánchez-Osuna, M; Casanelles, E; García-Belinchón, M; Comella, JX; Yuste, VJ
The Journal of biological chemistry
288
9200-15
2013
Mostrar resumen
Apoptotic nuclear morphology and oligonucleosomal double-strand DNA fragments (also known as DNA ladder) are considered the hallmarks of apoptotic cell death. From a classic point of view, these two processes occur concomitantly. Once activated, DNA fragmentation factor, 40-kDa subunit (DFF40)/caspase-activated DNase (CAD) endonuclease hydrolyzes the DNA into oligonucleosomal-size pieces, facilitating the chromatin package. However, the dogma that the apoptotic nuclear morphology depends on DNA fragmentation has been questioned. Here, we use different cellular models, including MEF CAD(-/-) cells, to unravel the mechanism by which DFF40/CAD influences chromatin condensation and nuclear collapse during apoptosis. Upon apoptotic insult, SK-N-AS cells display caspase-dependent apoptotic nuclear alterations in the absence of internucleosomal DNA degradation. The overexpression of a wild-type form of DFF40/CAD endonuclease, but not of different catalytic-null mutants, restores the cellular ability to degrade the chromatin into oligonucleosomal-length fragments. We show that apoptotic nuclear collapse requires a 3'-OH endonucleolytic activity even though the internucleosomal DNA degradation is impaired. Moreover, alkaline unwinding electrophoresis and In Situ End-Labeling (ISEL)/In Situ Nick Translation (ISNT) assays reveal that the apoptotic DNA damage observed in the DNA ladder-deficient SK-N-AS cells is characterized by the presence of single-strand nicks/breaks. Apoptotic single-strand breaks can be impaired by DFF40/CAD knockdown, abrogating nuclear collapse and disassembly. In conclusion, the highest order of chromatin compaction observed in the later steps of caspase-dependent apoptosis relies on DFF40/CAD-mediated DNA damage by generating 3'-OH ends in single-strand rather than double-strand DNA nicks/breaks. | 23430749
 |
Apoptotic DNA degradation into oligonucleosomal fragments, but not apoptotic nuclear morphology, relies on a cytosolic pool of DFF40/CAD endonuclease.
Iglesias-Guimarais, V; Gil-Guiñon, E; Gabernet, G; García-Belinchón, M; Sánchez-Osuna, M; Casanelles, E; Comella, JX; Yuste, VJ
The Journal of biological chemistry
287
7766-79
2011
Mostrar resumen
Apoptotic cell death is characterized by nuclear fragmentation and oligonucleosomal DNA degradation, mediated by the caspase-dependent specific activation of DFF40/CAD endonuclease. Here, we describe how, upon apoptotic stimuli, SK-N-AS human neuroblastoma-derived cells show apoptotic nuclear morphology without displaying concomitant internucleosomal DNA fragmentation. Cytotoxicity afforded after staurosporine treatment is comparable with that obtained in SH-SY5Y cells, which exhibit a complete apoptotic phenotype. SK-N-AS cell death is a caspase-dependent process that can be impaired by the pan-caspase inhibitor q-VD-OPh. The endogenous inhibitor of DFF40/CAD, ICAD, is correctly processed, and dff40/cad cDNA sequence does not reveal mutations altering its amino acid composition. Biochemical approaches show that both SH-SY5Y and SK-N-AS resting cells express comparable levels of DFF40/CAD. However, the endonuclease is poorly expressed in the cytosolic fraction of healthy SK-N-AS cells. Despite this differential subcellular distribution of DFF40/CAD, we find no differences in the subcellular localization of both pro-caspase-3 and ICAD between the analyzed cell lines. After staurosporine treatment, the preferential processing of ICAD in the cytosolic fraction allows the translocation of DFF40/CAD from this fraction to a chromatin-enriched one. Therefore, the low levels of cytosolic DFF40/CAD detected in SK-N-AS cells determine the absence of DNA laddering after staurosporine treatment. In these cells DFF40/CAD cytosolic levels can be restored by the overexpression of their own endonuclease, which is sufficient to make them proficient at degrading their chromatin into oligonucleosome-size fragments after staurosporine treatment. Altogether, the cytosolic levels of DFF40/CAD are determinants in achieving a complete apoptotic phenotype, including oligonucleosomal DNA degradation. | 22253444
 |
EndoG links Bnip3-induced mitochondrial damage and caspase-independent DNA fragmentation in ischemic cardiomyocytes.
Zhang, J; Ye, J; Altafaj, A; Cardona, M; Bahi, N; Llovera, M; Cañas, X; Cook, SA; Comella, JX; Sanchis, D
PloS one
6
e17998
2010
Mostrar resumen
Mitochondrial dysfunction, caspase activation and caspase-dependent DNA fragmentation are involved in cell damage in many tissues. However, differentiated cardiomyocytes repress the expression of the canonical apoptotic pathway and their death during ischemia is caspase-independent. The atypical BH3-only protein Bnip3 is involved in the process leading to caspase-independent DNA fragmentation in cardiomyocytes. However, the pathway by which DNA degradation ensues following Bnip3 activation is not resolved. To identify the mechanism involved, we analyzed the interdependence of Bnip3, Nix and EndoG in mitochondrial damage and DNA fragmentation during experimental ischemia in neonatal rat ventricular cardiomyocytes. Our results show that the expression of EndoG and Bnip3 increases in the heart throughout development, while the caspase-dependent machinery is silenced. TUNEL-positive DNA damage, which depends on caspase activity in other cells, is caspase-independent in ischemic cardiomyocytes and ischemia-induced DNA high and low molecular weight fragmentation is blocked by repressing EndoG expression. Ischemia-induced EndoG translocation and DNA degradation are prevented by silencing the expression of Bnip3, but not Nix, or by overexpressing Bcl-x(L). These data establish a link between Bnip3 and EndoG-dependent, TUNEL-positive, DNA fragmentation in ischemic cardiomyocytes in the absence of caspases, defining an alternative cell death pathway in postmitotic cells. Artículo Texto completo | 21437288
 |
AMPA-induced excitotoxicity increases nuclear levels of CAD, endonuclease G, and acinus and induces chromatin condensation in rat hippocampal pyramidal neurons.
W M Henne, S Oomman, J Attridge, V Finckbone, P Coates, R Bliss, H Strahlendorf, J Strahlendorf
Cellular and molecular neurobiology
26
321-39
2005
Mostrar resumen
Programmed cell death has been linked to AMPA-receptor-mediated excitotoxicity in pyramidal neurons of the hippocampus. The intent of this study was to investigate the roles of caspase-dependent and independent nuclear death-related factors in mediating AMPA-induced nuclear changes in PyNs by use of immunohistochemistry and transmission electron microscopy (TEM). Data indicate increases in the nuclear levels of caspase-activated acinus and DNase and Endonuclease G (a caspase-independent endonuclease) in CA1 and CA3 PyN nuclei with different temporal patterns following an AMPA-insult. Hoechst staining and TEM confirm AMPA-induced chromatin condensation. The presence of active acinus in nuclei suggests it mediates chromatin condensation. Interestingly, a DNA fragmentation labeling protocol showed that there was no chromatin cleavage up to 90 min after AMPA-insult. Overall, we conclude that: 1) AMPA-induced excitotoxicity increases nuclear immunoreactivity of pro-death enzymes from multiple programmed cell death pathways, 2) differential chromatin condensation patterns occur between CA1 and CA3, and 3) there is no chromatin cleavage within our experimental timeframe. | 16767516
 |
Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis.
Sakahira, H, et al.
Nature, 391: 96-9 (1998)
1998
Mostrar resumen
Various molecules such as cytokines and anticancer drugs, as well as factor deprivation, rapidly induce apoptosis (programmed cell death), which is morphologically characterized by cell shrinkage and the blebbing of plasma membranes and by nuclear condensation. Caspases, particularly caspase 3, are proteases that are activated during apoptosis and which cleave substrates such as poly(ADP-ribose) polymerase, actin, fodrin, and lamin. Apoptosis is also accompanied by the internucleosomal degradation of chromosomal DNA. In the accompanying Article, we have identified and molecularly cloned a caspase-activated deoxyribonuclease (CAD) and its inhibitor (ICAD). Here we show that caspase 3 cleaves ICAD and inactivates its CAD-inhibitory effect. We identified two caspase-3 cleavage sites in ICAD by site-directed mutagenesis. When human Jurkat cells were transformed with ICAD-expressing plasmid, occupation of the receptor Fas, which normally triggers apoptosis, did not result in DNA degradation. The ICAD transformants were also resistant to staurosporine-induced DNA degradation, although staurosporine still killed the cells by activating caspase. Our results indicate that activation of CAD downstream of the caspase cascade is responsible for internucleosomal DNA degradation during apoptosis, and that ICAD works as an inhibitor of this process. | 9422513
 |
Molecular cloning and characterization of human caspase-activated DNase
Mukae, N, et al
Proc Natl Acad Sci USA, 95:9123-8 (1998)
1998
| 9689044
 |
Involvement of an ICE-like protease in Fas-mediated apoptosis.
Enari, M, et al.
Nature, 375: 78-81 (1995)
1994
Mostrar resumen
Fas is a type-I membrane protein that transduces an apoptotic signal. Binding of Fas ligand or agonistic anti-Fas antibody to Fas kills the cells by apoptosis. Studies in the nematode Caenorhabditis elegans have suggested that proteases such as interleukin-1 beta-converting enzyme (ICE) or the product of the C. elegans cell-death gene ced-3 are involved in apoptotic signal transduction. The activity of ICE can be inhibited by the product of crmA, a cytokine-response modifier gene encoded by cowpox virus. We report here that expression of crmA inhibits cytotoxicity induced by anti-Fas antibody or tumour necrosis factor (TNF). We have found a specific ICE inhibitor tetrapeptide (acetyl-Tyr-Val-Ala-Asp-chloromethylketone) that also prevents apoptosis induced by anti-Fas antibody. These results suggest an involvement of an ICE-like protease in Fas-mediated apoptosis and TNF-induced cytotoxicity. | 7536900
 |