Two nested developmental waves demarcate a compartment boundary in the mouse lung.
Alanis, DM; Chang, DR; Akiyama, H; Krasnow, MA; Chen, J
Nature communications
5
3923
2014
Mostrar resumen
The lung is a branched tubular network with two distinct compartments--the proximal conducting airways and the peripheral gas exchange region--separated by a discrete boundary termed the bronchoalveolar duct junction (BADJ). Here we image the developing mouse lung in three-dimensions (3D) and show that two nested developmental waves demarcate the BADJ under the control of a global hormonal signal. A first wave of branching morphogenesis progresses throughout embryonic development, generating branches for both compartments. A second wave of conducting airway differentiation follows the first wave but terminates earlier, specifying the proximal compartment and setting the BADJ. The second wave is terminated by a glucocorticoid signalling: premature activation or loss of glucocorticoid signalling causes a proximal or distal shift, respectively, in BADJ location. The results demonstrate a new mechanism of boundary formation in complex, 3D organs and provide new insights into glucocorticoid therapies for lung defects in premature birth. | | 24879355
 |
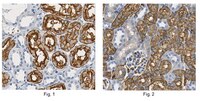
Expression of E-cadherin in pig kidney.
Lee, SY; Han, SM; Kim, JE; Chung, KY; Han, KH
Journal of veterinary science
14
381-6
2013
Mostrar resumen
E-cadherin is a cell adhesion molecule that plays an important role in maintaining renal epithelial polarity and integrity. The purpose of this study was to determine the exact cellular localization of E-cadherin in pig kidney. Kidney tissues from pigs were processed for light and electron microscopy immunocytochemistry, and immunoblot analysis. E-cadhedrin bands of the same size were detected by immunoblot of samples from rat and pig kidneys. In pig kidney, strong E-cadherin expression was observed in the basolateral plasma membrane of the tubular epithelial cells. E-cadherin immunolabeling was not detected in glomeruli or blood vessels of pig kidney. Double-labeling results demonstrated that E-cadherin was expressed in the calbindin D28k-positive distal convoluted tubule and H(+)-ATPase- positive collecting duct, but not in the aquaporin 1-positive, N-cadherin-positive proximal tubule. In contrast to rat, E-cadherin immunoreactivity was not expressed at detectable levels in the Tamm-Horsfall protein-positive thick ascending limb of pig kidney. Immunoelectron microscopy confirmed that E-cadherin was localized in both the lateral membranes and basal infoldings of the collecting duct. These results suggest that E-cadherin may be a critical adhesion molecule in the distal convoluted tubule and collecting duct cells of pig kidney. | | 23820247
 |
Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury.
Zhou, D; Tan, RJ; Lin, L; Zhou, L; Liu, Y
Kidney international
84
509-20
2013
Mostrar resumen
Hepatocyte growth factor is a pleiotrophic protein that promotes injury repair and regeneration in multiple organs. Here, we show that after acute kidney injury (AKI), the HGF receptor, c-met, was induced predominantly in renal tubular epithelium. To investigate the role of tubule-specific induction of c-met in AKI, we generated conditional knockout mice, in which the c-met gene was specifically disrupted in renal tubules. These Ksp-met-/- mice were phenotypically normal and had no appreciable defect in kidney morphology and function. However, in AKI induced by cisplatin or ischemia/reperfusion injury, the loss of tubular c-met substantially aggravated renal injury. Compared with controls, Ksp-met-/- mice displayed higher serum creatinine, more severe morphologic lesions, and increased apoptosis, which was accompanied by an increased expression of Bax and Fas ligand and decreased phosphorylation/activation of Akt. In addition, ablation of c-met in renal tubules promoted chemokine expression and renal inflammation after AKI. Consistently, ectopic expression of hepatocyte growth factor in vivo protected the kidneys against AKI in control mice, but not in Ksp-met-/- counterparts. Thus, our results suggest that tubule-specific c-met signaling is crucial in conferring renal protection after AKI, primarily by its anti-apoptotic and anti-inflammatory mechanisms. | | 23715119
 |
Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling.
Zhou, L; Li, Y; Zhou, D; Tan, RJ; Liu, Y
Journal of the American Society of Nephrology : JASN
24
771-85
2013
Mostrar resumen
Aging is an independent risk factor for CKD, but the molecular mechanisms that link aging and CKD are not well understood. The antiaging protein Klotho may be an endogenous antagonist of Wnt/β-catenin signaling, which promotes fibrogenesis, suggesting that loss of Klotho may contribute to CKD through increased Wnt/β-catenin activity. Here, normal adult kidneys highly expressed Klotho in the tubular epithelium, but various models of nephropathy exhibited markedly less expression of Klotho. Loss of Klotho was closely associated with increased β-catenin in the diseased kidneys, suggesting an inverse correlation between Klotho and canonical Wnt signaling. In vitro, both full-length and secreted Klotho bound to multiple Wnts, including Wnt1, Wnt4, and Wnt7a. Klotho repressed gene transcription induced by Wnt but not by active β-catenin. Furthermore, Klotho blocked Wnt-triggered activation and nuclear translocation of β-catenin, as well as the expression of its target genes in tubular epithelial cells. Investigating potential mediators of Klotho loss in CKD, we found that TGF-β1 suppressed Klotho expression and concomitantly activated β-catenin; conversely, overexpression of Klotho abolished fibrogenic effects of TGF-β1. In two mouse models of CKD induced by unilateral ureteral obstruction or adriamycin, in vivo expression of secreted Klotho inhibited the activation of renal β-catenin and expression of its target genes. Secreted Klotho also suppressed myofibroblast activation, reduced matrix expression, and ameliorated renal fibrosis. Taken together, these results suggest that Klotho is an antagonist of endogenous Wnt/β-catenin activity; therefore, loss of Klotho may contribute to kidney injury by releasing the repression of pathogenic Wnt/β-catenin signaling. | | 23559584
 |
Lung epithelial branching program antagonizes alveolar differentiation.
Chang, DR; Martinez Alanis, D; Miller, RK; Ji, H; Akiyama, H; McCrea, PD; Chen, J
Proceedings of the National Academy of Sciences of the United States of America
110
18042-51
2013
Mostrar resumen
Mammalian organs, including the lung and kidney, often adopt a branched structure to achieve high efficiency and capacity of their physiological functions. Formation of a functional lung requires two developmental processes: branching morphogenesis, which builds a tree-like tubular network, and alveolar differentiation, which generates specialized epithelial cells for gas exchange. Much progress has been made to understand each of the two processes individually; however, it is not clear whether the two processes are coordinated and how they are deployed at the correct time and location. Here we show that an epithelial branching morphogenesis program antagonizes alveolar differentiation in the mouse lung. We find a negative correlation between branching morphogenesis and alveolar differentiation temporally, spatially, and evolutionarily. Gain-of-function experiments show that hyperactive small GTPase Kras expands the branching program and also suppresses molecular and cellular differentiation of alveolar cells. Loss-of-function experiments show that SRY-box containing gene 9 (Sox9) functions downstream of Fibroblast growth factor (Fgf)/Kras to promote branching and also suppresses premature initiation of alveolar differentiation. We thus propose that lung epithelial progenitors continuously balance between branching morphogenesis and alveolar differentiation, and such a balance is mediated by dual-function regulators, including Kras and Sox9. The resulting temporal delay of differentiation by the branching program may provide new insights to lung immaturity in preterm neonates and the increase in organ complexity during evolution. | Immunohistochemistry | 24058167
 |
A new AQP1 null allele identified in a Gypsy woman who developed an anti-CO3 during her first pregnancy.
Saison, C; Peyrard, T; Landre, C; Ballif, BA; Schlosser, KA; Dettori, I; Chicheportiche, C; Nemeth, P; Cartron, JP; Arnaud, L
Vox sanguinis
103
137-44
2011
Mostrar resumen
The Colton blood group antigens are carried by the AQP1 water channel. AQP1(-/-) individuals, also known as Colton-null since they express no Colton antigens, do not suffer any apparent clinical consequence but may develop a clinically significant alloantibody (anti-CO3) induced by transfusion or pregnancy. Identification and transfusion support of Colton-null patients are highly challenging, not only due to the extreme rarity of this phenotype, the lack of appropriate reagents in most laboratories, as well as the possibility of confusing it with the recently described CO:-1,-2,3,-4 phenotype where AQP1 is present. This study investigated a new Colton-null case and evaluated three commercially available anti-AQP1s to identify Colton-null red blood cell samples.The Colton-null phenotype was investigated by standard serological techniques, AQP1 sequencing, immunoblot and flow cytometry analyses.We identified and characterized the Colton-null phenotype in a Gypsy woman who developed an anti-CO3 during her first pregnancy. After developing a simple and robust method to sequence AQP1, we showed that she was apparently homozygous for a new AQP1 null allele, AQP1 601delG, whose product is not expressed in her red blood cells. We also established the Colton specificity of three commercially available anti-AQP1s in immunoblot and/or flow cytometry analyses.This Gypsy woman represents the sixth Colton-null case characterized at the serological, genetic and biochemical levels. The validation here of new reagents and methods should facilitate the identification of Colton-null individuals. | | 22348807
 |
Dissociation of embryonic kidney followed by re-aggregation as a method for chimeric analysis.
Jamie A Davies,Mathieu Unbekandt,Jessica Ineson,Michael Lusis,Melissa H Little
Methods in molecular biology (Clifton, N.J.)
886
2011
Mostrar resumen
This chapter presents three methods for re-constructing mouse foetal kidney tissue from simple suspensions of cells. These techniques are very useful for a number of purposes: (1) they allow the production of fine-grained chimaeras in which cell autonomy of mutations can be tested, (2) they provide an environment that allows the renal differentiation potential of stem cells to be assessed, and (3) they are an excellent system in which to study the mechanisms of self-organization. Each of the methods described here begins with disaggregation of embryonic mouse kidneys, followed by re-aggregation and culture; the main differences are in the culture methods, each of which has advantages for particular purposes. | | 22639257
 |
BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors.
Watanabe, M; Kang, YJ; Davies, LM; Meghpara, S; Lau, K; Chung, CY; Kathiriya, J; Hadjantonakis, AK; Monuki, ES
The Journal of neuroscience : the official journal of the Society for Neuroscience
32
15934-45
2011
Mostrar resumen
Choroid plexus epithelial cells (CPECs) have essential developmental and homeostatic roles related to the CSF and blood-CSF barrier they produce. Accordingly, CPEC dysfunction has been implicated in many neurological disorders, such as Alzheimer's disease, and transplant studies have provided proof-of-concept for CPEC-based therapies. However, such therapies have been hindered by the inability to expand or generate CPECs in culture. During development, CPECs differentiate from preneurogenic neuroepithelial cells and require bone morphogenetic protein (BMP) signaling, but whether BMPs suffice for CPEC induction is unknown. Here we provide evidence for BMP4 sufficiency to induce CPEC fate from neural progenitors derived from mouse embryonic stem cells (ESCs). CPEC specification by BMP4 was restricted to an early time period after neural induction in culture, with peak CPEC competency correlating to neuroepithelial cells rather than radial glia. In addition to molecular, cellular, and ultrastructural criteria, derived CPECs (dCPECs) had functions that were indistinguishable from primary CPECs, including self-assembly into secretory vesicles and integration into endogenous choroid plexus epithelium following intraventricular injection. We then used BMP4 to generate dCPECs from human ESC-derived neuroepithelial cells. These findings demonstrate BMP4 sufficiency to instruct CPEC fate, expand the repertoire of stem cell-derived neural derivatives in culture, and herald dCPEC-based therapeutic applications aimed at the unique interface between blood, CSF, and brain governed by CPECs. | Immunofluorescence | 23136431
 |
Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice.
Zhou, D; Li, Y; Lin, L; Zhou, L; Igarashi, P; Liu, Y
Kidney international
82
537-47
2011
Mostrar resumen
β-Catenin is a unique intracellular protein functioning as an integral component of the cell-cell adherens complex and a principal signaling protein mediating canonical Wnt signaling. Little is known about its function in adult kidneys in the normal physiologic state or after acute kidney injury (AKI). To study this, we generated conditional knockout mice in which the β-catenin gene was specifically disrupted in renal tubules (Ksp-β-cat-/-). These mice were phenotypically normal with no appreciable defects in kidney morphology and function. In the absence of β-catenin, γ-catenin functionally substituted for it in E-cadherin binding, thereby sustaining the integrity of epithelial adherens junctions in the kidneys. In AKI induced by ischemia reperfusion or folic acid, the loss of tubular β-catenin substantially aggravated renal lesions. Compared with controls, Ksp-β-cat-/- mice displayed higher mortality, elevated serum creatinine, and more severe morphologic injury. Consistently, apoptosis was more prevalent in kidneys of the knockout mice, which was accompanied by increased expression of p53 and Bax, and decreased phosphorylated Akt and survivin. In vitro activation of β-catenin by Wnt1 or stabilization of β-catenin protected tubular epithelial cells from apoptosis, activated Akt, induced survivin, and repressed p53 and Bax expression. Hence, endogenous β-catenin is pivotal for renal tubular protection after AKI by promoting cell survival through multiple mechanisms. | Immunohistochemistry | 22622501
 |
Fluid-percussion brain injury induces changes in aquaporin channel expression.
Oliva AA Jr, Kang Y, Truettner JS, Sanchez-Molano J, Furones C, Yool AJ, Atkins CM
Neuroscience
2010
Mostrar resumen
Edema, the accumulation of excess fluid, is a major pathological change in the brain that contributes significantly to pathology and mortality after moderate to severe brain injury. Edema is regulated by aquaporin (AQP) channels which transport water across cellular membranes. Six AQPs are found in the brain (1, 3, 4, 5, 8, and 9), and previous studies have found that AQP4 is regulated after traumatic brain injury (TBI). To further understand how AQPs contribute to brain edema, we investigated whether expression of AQP1, 3, and 9 are also regulated after TBI. Adult male Sprague Dawley rats received moderate parasagittal fluid-percussion brain injury (FPI) or sham surgery. After induction of FPI, the injured, ipsilateral parietal cortex and hippocampus were dissected and analyzed by Western blotting. We observed a small decrease in AQP3 and 4 levels at 7 days after FPI in the ipsilateral, parietal cortex. Both AQP1 and 9 significantly increased within 30 min post-injury and remained elevated for up to 6 h in the ipsilateral, parietal cortex. Aqp1 and 9 mRNA levels were also significantly increased at 30 min post-FPI. Administration of an AQP1 and 4 antagonist, AqB013, non-significantly increased brain water content in sham, non-injured animals, and did not prevent edema formation 24 h after trauma in either the parietal cortex or hippocampus. These results indicate that Aqp1 and 9 mRNA and protein levels increase after moderate parasagittal FPI and that an inhibitor of AQP1 and 4 does not decrease edema after moderate parasagittal FPI.Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved. | | 21329742
 |