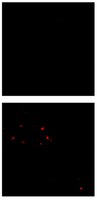
MABN1839 Sigma-AldrichAnti-Amyloid-β (oligomer) Antibody, clone F11G3
This Anti-Amyloid-β (oligomer) Antibody, clone F11G3 is validated for use in Western Blotting, Dot Blot, ELISA, Immunofluorescence, Immunoprecipitation for the detection of Amyloid-β.
More>> This Anti-Amyloid-β (oligomer) Antibody, clone F11G3 is validated for use in Western Blotting, Dot Blot, ELISA, Immunofluorescence, Immunoprecipitation for the detection of Amyloid-β. Less<<Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| Species Reactivity | Key Applications | Host | Format | Antibody Type |
|---|---|---|---|---|
| H, M | WB, DB, ELISA, IF, IP | M | Purified | Monoclonal Antibody |
| References |
|---|
| Product Information | |
|---|---|
| Format | Purified |
| Presentation | Purified mouse monoclonal IgMκ antibody in PBS without preservatives. |
| Quality Level | MQ100 |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 100 μg |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| MABN1839 | 04055977314977 |
Documentation
Anti-Amyloid-β (oligomer) Antibody, clone F11G3 Ficha datos de seguridad (MSDS)
| Título |
|---|
Anti-Amyloid-β (oligomer) Antibody, clone F11G3 Certificados de análisis
Folleto
| Cargo |
|---|
| Neuroscience Solutions for productive research |