104833 Sigma-AldrichAck1 Inhibitor, AIM-100 - CAS 873305-35-2 - Calbiochem
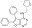
Sinónimos: Activated Cdc42 Kinase 1 Inhibitor, (S)-5,6-Diphenyl-N-((tetrahydrofuran-2-yl)methyl)furo[2,3-d]pyrimidin-4-amine
Productos recomendados
Descripción
| Replacement Information |
|---|
Tabla espec. clave
| CAS # | Empirical Formula |
|---|---|
| 873305-35-2 | C₂₃H₂₁N₃O₂ |
| Product Information | |
|---|---|
| CAS number | 873305-35-2 |
| Form | Yellow solid |
| Hill Formula | C₂₃H₂₁N₃O₂ |
| Chemical formula | C₂₃H₂₁N₃O₂ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Número de referencia | GTIN |
| 104833 | 0 |
Documentation
Referencias bibliográficas
| Visión general referencias |
|---|
| Mahajan, K., et al. 2012. J. Biol. Chem. 287, 22112. Mahajan, K., et al. 2012. Am. J. Pathol. 180, 1386. Mahajan, K., et al. 2010. Prostate 70, 1274. DiMauro, E.F., et al. 2007. Bioorg. Med. Chem. Lett. 17, 2305. |