RLI1MAG-92K MilliporeMILLIPLEX MAP Rat Liver Injury Panel - Toxicity Multiplex Assay
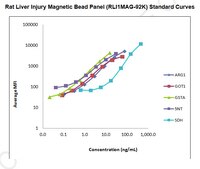
The analytes available for this multiplex kit are: Liver-type arginase 1 (ARG1), aspartate transaminase 1 (GOT1), α-glutathianone S-transferase (GSTα), sorbitol dehydrogenase (SDH), and 5'-Nucleotidase (5'-NT/CD73).
More>> The analytes available for this multiplex kit are: Liver-type arginase 1 (ARG1), aspartate transaminase 1 (GOT1), α-glutathianone S-transferase (GSTα), sorbitol dehydrogenase (SDH), and 5'-Nucleotidase (5'-NT/CD73). Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Analytes Available | Species Reactivity | Key Applications | Detection Methods |
|---|---|---|---|
| ARG1 GOT1 GSTα SDH 5'-NT/CD73 | R | Mplex | Luminex xMAP |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Detection method | Luminex xMAP |
| Configuration | Design your multiplex kit by choosing available analytes within this panel. |
| Precision, % |
|
| Panel Type | MAGNETIC Toxicity |
| Quality Level | MQ200 |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Storage and Shipping Information | |
|---|---|
| Storage Conditions | Recommended storage for kit components is 2 - 8°C. |
| Packaging Information | |
|---|---|
| Material Size | 96-well plate |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| RLI1MAG-92K | 04055977130737 |
Documentation
Protocols
| Title |
|---|
| Protocol- RLI1MAG-92K |
MILLIPLEX MAP Rat Liver Injury Panel - Toxicity Multiplex Assay SDS
| Title |
|---|
Brochure
| Title |
|---|
| NPI Flyer- Epigenetics and Nuclear Function Feature |
| New Products - Antibodies, Small Molecule, Inhibitors |
| The Power of Biomarker Analysis |
Technical Info
Posters
| Title |
|---|
| Toxicology in drug discovery and development: Biomarkers and model organisms |