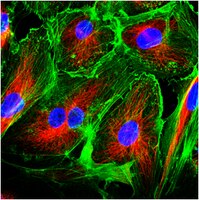
RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation.
Nakaya, Y; Sukowati, EW; Wu, Y; Sheng, G
Nature cell biology
10
765-75
2008
Show Abstract
Molecular and cellular mechanisms of epithelial-mesenchymal transition (EMT), crucial in development and pathogenesis, are still poorly understood. Here we provide evidence that distinct cellular steps of EMT occur sequentially during gastrulation. Basement membrane (BM) breakdown is the first recognizable step and is controlled by loss of basally localized RhoA activity and its activator neuroepithelial-transforming-protein-1 (Net1). Failure of RhoA downregulation during EMT leads to BM retention and reduction of its activity in normal epithelium leads to BM breakdown. We also show that this is in part mediated by RhoA-regulated basal microtubule stability. Microtubule disruption causes BM breakdown and its stabilization results in BM retention. We propose that loss of Net1 before EMT reduces basal RhoA activity and destabilizes basal microtubules, causing disruption of epithelial cell-BM interaction and subsequently, breakdown of the BM. | 18552836
 |
Tau aggregation and toxicity in a cell culture model of tauopathy.
Bandyopadhyay, B; Li, G; Yin, H; Kuret, J
The Journal of biological chemistry
282
16454-64
2007
Show Abstract
Intracellular aggregation of the microtubule-associated protein tau into filamentous inclusions is a defining characteristic of Alzheimer disease. Because appearance of tau-aggregate bearing lesions correlates with both cognitive decline and neurodegeneration, it has been hypothesized that tau aggregation may be directly toxic to cells that harbor them. Testing this hypothesis in cell culture has been complicated by the resistance of full-length tau isoforms to aggregation over experimentally tractable time periods. To overcome this limitation, a small-molecule agonist of the tau aggregation reaction, Congo red, was used to drive aggregation within HEK-293 cells expressing full-length tau isoform htau40. Formation of detergent-insoluble aggregates was both time and agonist concentration dependent. At 10 microM Congo red, detergent-insoluble aggregates appeared with pseudo-first order kinetics and a half-life of approximately 5 days. By 7 days in culture, total tau levels increased 2-fold, with approximately 30% of total tau converted into detergent-insoluble aggregates. Agonist addition also led to rapid losses in the tubulin binding activity of tau, although tau was not hyperphosphorylated as judged by occupancy of phosphorylation sites Ser396/Ser404. Tau aggregation was associated with decreased viability as detected by ToPro-3 uptake. The results, which establish a new approach for analysis of tau aggregation in cells independent of tau hyperphosphorylation, suggest that conformational changes associated with aggregation are incompatible with microtubule binding, and that toxicity associated with intracellular tau aggregation is not acute but develops over a period of days. | 17428800
 |
Amino acid sequence requirements in the epitope recognized by the alpha-tubulin-specific rat monoclonal antibody YL 1/2.
Wehland, J; Schröder, HC; Weber, K
The EMBO journal
3
1295-300
1984
Show Abstract
We have characterized the epitope of the rat monoclonal antibody YL 1/2 in detail using synthetic peptides and several alpha-tubulin derivatives. The epitope seems to be provided by the linear sequence spanning the carboxy-terminal residues of tyrosinated alpha-tubulin. By competitive ELISA, dipeptides covering the carboxyl end could be antigenically recognized. Three sites were deduced at the dipeptide level: a negatively charged side chain in the penultimate position followed by an aromatic residue which must carry the free carboxylate group. Experiments with longer peptides point to a further negative charge provided by a carboxylate group on the third residue from the end. Thus the tripeptide Glu-Glu-Tyr was only 5-fold less active than the octapeptide spanning the carboxy-terminal alpha-tubulin sequence. The octapeptide itself showed only a 40-fold lower activity than tyrosinated alpha-tubulin. In line with the emerging epitope requirements of YL 1/2, the Escherichia coli rec A protein, the catalytic subunit of the cyclic AMP-dependent muscle protein kinase as well as performic acid-oxidized actin were recognized by YL 1/2 in immunoblots. These results thus define the sequence requirements within a probably linear epitope and give rise to some general questions concerning experiments where monoclonal antibodies are microinjected into cells in order to assess the contribution of a known antigen to cellular physiology. | 6204858
 |
A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. II. Effects on cell movement, organization of microtubules, and intermediate filaments, and arrangement of Golgi elements.
Wehland, J; Willingham, MC
The Journal of cell biology
97
1476-90
1983
Show Abstract
A rat monoclonal antibody against yeast alpha-tubulin (clone YL 1/2; Kilmartin, J. V., B. Wright, and C. Milstein, 1982, J. Cell Biol., 93:576-582) that reacts specifically with the tyrosylated form of alpha-tubulin and readily binds to tubulin in microtubules when injected into cultured cells (see Wehland, J., M. C. Willingham, and I. V. Sandoval, 1983, J. Cell Biol., 97:1467-1475) was used to study microtubule organization and function in living cells. Depending on the concentration of YL 1/2 that was injected the following striking effects were observed: (a) When injected at a low concentration (2 mg IgG/ml in the injection solution), where microtubules were decorated without changing their distribution, intracellular movement of cell organelles (saltatory movement) and cell translocation were not affected. Intermediate concentrations (6 mg IgG/ml) that induced bundling but no perinuclear aggregation of microtubules abolished saltatory movement and cell translocation, and high concentrations (greater than 12 mg IgG/ml) that induced perinuclear aggregation of microtubules showed the same effect. (b) YL 1/2, when injected at intermediate and high concentrations, arrested cells in mitosis. Such cells showed no normal spindle structures. (c) Injection of an intermediate concentration of YL 1/2 that stopped saltatory movement caused little or no aggregation of intermediate filaments and no dispersion of the Golgi complex. After injection of high concentrations, resulting in perinuclear aggregation of microtubules, intermediate filaments formed perinuclear bundles and the Golgi complex became dispersed analogous to results obtained after treatment of cells with colcemid. (d) When rhodamine-conjugated YL 1/2 was injected at concentrations that stopped saltatory movement and arrested cells in mitosis, microtubule structures could be visualized and followed for several hours in living cells by video image intensification microscopy. They showed little or no change in distribution and organization during observation, even though these microtubule structures appeared not to be stabilized by injected YL 1/2 since they were readily depolymerized by colcemid or cold treatment and repolymerized upon drug removal or rewarming to 37 degrees C, respectively. These results are discussed in terms of the participation of microtubules in cellular activities such as cell movement and cytoplasmic organization and in terms of the specificity of YL 1/2 for the tyrosylated form of alpha-tubulin. | 6685128
 |
Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line.
Kilmartin, JV; Wright, B; Milstein, C
The Journal of cell biology
93
576-82
1982
Show Abstract
Hybrid myeloma cell lines secreting monoclonal antibodies to tubulin have been prepared using rat myelomas and spleen cells from rats immunized with yeast tubulin. A comparison between the results obtained with the rat myeloma Y3-Ag 1.2.3., which secretes a light chain, and a new line, YB2/O, which does not, shows that they are both excellent parental lines and that the second produces hybrids with no myeloma chain components. The antitubulin antibodies in the serum of rats bearing two of the hybrid myeloma tumors gave titers of up to 1:10(6) from which large amounts of monoclonal antibodies could be easily purified. They recognized tubulin from yeast as well as from birds and mammals. The two antibodies gave clear immunofluorescent staining of yeast mitotic spindles as well as the interphase microtubule network of tissue culture cells. Some difference in the pattern of immunofluorescence staining of yeast cells and nuclei was observed between the two antibodies. The purified antibodies could be conjugated to colloidal gold particles and used for direct labeling of yeast microtubules for electron microscopy. | 6811596
 |