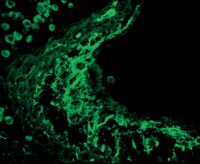
Expression of pigment epithelium-derived factor and thrombospondin-1 regulate proliferation and migration of retinal pigment epithelial cells.
Farnoodian, M; Kinter, JB; Yadranji Aghdam, S; Zaitoun, I; Sorenson, CM; Sheibani, N
Physiological reports
3
2015
Show Abstract
Age-related macular degeneration (AMD) is the leading cause of vision loss among elderly. Although the pathogenesis of AMD is associated with retinal pigmented epithelium (RPE) dysfunction and abnormal neovascularization the detailed mechanisms remain unresolved. RPE is a specialized monolayer of epithelial cells with important functions in ocular homeostasis. Pathological RPE damage contributes to major ocular conditions including retinal degeneration and irreversible loss of vision in AMD. RPE cells also assist in the maintenance of the ocular angiogenic balance by production of positive and negative regulatory factors including vascular endothelial growth factor (VEGF), thrombospondin-1 (TSP1), and pigment epithelium-derived factor (PEDF). The altered production of PEDF and TSP1, as endogenous inhibitors of angiogenesis and inflammation, by RPE cells have been linked to pathogenesis of AMD and choroidal and retinal neovascularization. However, lack of simple methods for isolation and culture of mouse RPE cells has resulted in limited knowledge regarding the cell autonomous role of TSP1 and PEDF in RPE cell function. Here, we describe a method for routine isolation and propagation of RPE cells from wild-type, TSP1, and PEDF-deficient mice, and have investigated their impact on RPE cell function. We showed that expression of TSP1 and PEDF significantly impacted RPE cell proliferation, migration, adhesion, oxidative state, and phagocytic activity with minimal effect on their basal rate of apoptosis. Together, our results indicated that the expression of PEDF and TSP1 by RPE cells play crucial roles not only in regulation of ocular vascular homeostasis but also have significant impact on their cellular function. | | | 25602019
 |
The impact of flow-induced forces on the morphogenesis of the outflow tract.
Biechler, SV; Junor, L; Evans, AN; Eberth, JF; Price, RL; Potts, JD; Yost, MJ; Goodwin, RL
Frontiers in physiology
5
225
2014
Show Abstract
One percent of infants are born with congenital heart disease (CHD), which commonly involves outflow tract (OFT) defects. These infants often require complex surgeries, which are associated with long term adverse remodeling effects, and receive replacement valves with limited strength, biocompatibility, and growth capability. To address these problematic issues, researchers have carried out investigations in valve development and valve mechanics. A longstanding hypothesis is that flow-induced forces regulate fibrous valve development, however, the specific mechanisms behind this mechanotransduction remain unclear. The purpose of this study was to implement an in vitro system of outflow tract development to test the response of embryonic OFT tissues to fluid flow. A dynamic, three-dimensional bioreactor system was used to culture embryonic OFT tissue under different levels of flow as well as the absence of flow. In the absence of flow, OFT tissues took on a more primitive phenotype that is characteristic of early OFT cushion development where widely dispersed mesenchymal cells are surrounded by a sparse, disorganized extracellular matrix (ECM). Whereas OFT tissues subjected to physiologically matched flow formed compact mounds of cells, initated, fibrous ECM development, while prolonged supraphysiological flow resulted in abnormal tissue remodeling. This study indicates that both the timing and magnitude of flow alter cellular processes that determine if OFT precursor tissue undergoes normal or pathological development. Specifically, these experiments showed that flow-generated forces regulate the deposition and localization of fibrous ECM proteins, indicating that mechanosensitive signaling pathways are capable of driving pathological OFT development if flows are not ideal. | Immunohistochemistry | | 24987377
 |
Expression of thrombospondin-1 modulates the angioinflammatory phenotype of choroidal endothelial cells.
Fei, P; Zaitoun, I; Farnoodian, M; Fisk, DL; Wang, S; Sorenson, CM; Sheibani, N
PloS one
9
e116423
2014
Show Abstract
The choroidal circulation plays a central role in maintaining the health of outer retina and photoreceptor function. Alterations in this circulation contribute to pathogenesis of many eye diseases including exudative age-related macular degeneration. Unfortunately, very little is known about the choroidal circulation and its molecular and cellular regulation. This has been further hampered by the lack of methods for routine culturing of choroidal endothelial cells (ChEC), especially from wild type and transgenic mice. Here we describe a method for isolation and culturing of mouse ChEC. We show that expression of thrombospondin-1 (TSP1), an endogenous inhibitor of angiogenesis and inflammation, has a significant impact on phenotype of ChEC. ChEC from TSP1-deficient (TSP1-/-) mice were less proliferative and more apoptotic, less migratory and less adherent, and failed to undergo capillary morphogenesis in Matrigel. However, re-expression of TSP1 was sufficient to restore TSP1-/- ChEC migration and capillary morphogenesis. TSP1-/- ChEC expressed increased levels of TSP2, phosphorylated endothelial nitric oxide synthase (NOS) and inducible NOS (iNOS), a marker of inflammation, which was associated with significantly higher level of NO and oxidative stress in these cells. Wild type and TSP1-/- ChEC produced similar levels of VEGF, although TSP1-/- ChEC exhibited increased levels of VEGF-R1 and pSTAT3. Other signaling pathways including Src, Akt, and MAPKs were not dramatically affected by the lack of TSP1. Together our results demonstrate an important autocrine role for TSP1 in regulation of ChEC phenotype. | Western Blotting | | 25548916
 |
Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise.
Hyldahl, RD; Olson, T; Welling, T; Groscost, L; Parcell, AC
Frontiers in physiology
5
485
2014
Show Abstract
Optimal repair and adaptation of skeletal muscle is facilitated by resident stem cells (satellite cells). To understand how different exercise modes influence satellite cell dynamics, we measured satellite cell activity in conjunction with markers of muscle damage and inflammation in human skeletal muscle following a single work- and intensity-matched bout of eccentric (ECC) or concentric contractions (CON). Participants completed a single bout of ECC (n = 7) or CON (n = 7) of the knee extensors. A muscle biopsy was obtained before and 24 h after exercise. Functional measures and immunohistochemical analyses were used to determine the extent of muscle damage and indices of satellite cell activity. Cytokine concentrations were measured using a multiplexed magnetic bead assay. Isokinetic peak torque decreased following ECC (p less than 0.05) but not CON. Greater histological staining of the damage marker Xin was observed in muscle samples of ECC vs. CON. Tenasin C immunoreactivity increased 15 fold (p less than 0.01) following ECC and was unchanged following CON. The inflammatory cytokines interferon gamma-induced protein 10 (IP-10) and monocyte chemotactic protein 1 (MCP-1) increased pre- to post-ECC (4.26 ± 1.4 vs. 10.49 ± 5.8 pg/ml, and 3.06 ± 0.7 vs. 6.25 ± 4.6 pg/ml, respectively; p less than 0.05). There was no change in any cytokine post-CON. Satellite cell content increased 27% pre- to post-ECC (0.10 ± 0.031 vs. 0.127 ± 0.041, respectively; p less than 0.05). There was no change in satellite cell number in CON (0.099 ± 0.027 vs. 0.102 ± 0.029, respectively). There was no fiber type-specific satellite cell response following either exercise mode. ECC but not CON resulted in an increase in MyoD positive nuclei per myofiber pre- to post-exercise (p less than 0.05), but there was no change in MyoD DNA binding activity in either condition. In conclusion, ECC but not CON results in functional and histological evidence of muscle damage that is accompanied by increased satellite cell activity 24 h post-exercise. | | | 25566087
 |
Development of a reactive stroma associated with prostatic intraepithelial neoplasia in EAF2 deficient mice.
Pascal, LE; Ai, J; Masoodi, KZ; Wang, Y; Wang, D; Eisermann, K; Rigatti, LH; O'Malley, KJ; Ma, HM; Wang, X; Dar, JA; Parwani, AV; Simons, BW; Ittman, MM; Li, L; Davies, BJ; Wang, Z
PloS one
8
e79542
2013
Show Abstract
ELL-associated factor 2 (EAF2) is an androgen-responsive tumor suppressor frequently deleted in advanced prostate cancer that functions as a transcription elongation factor of RNA Pol II through interaction with the ELL family proteins. EAF2 knockout mice on a 129P2/OLA-C57BL/6J background developed late-onset lung adenocarcinoma, hepatocellular carcinoma, B-cell lymphoma and high-grade prostatic intraepithelial neoplasia. In order to further characterize the role of EAF2 in the development of prostatic defects, the effects of EAF2 loss were compared in different murine strains. In the current study, aged EAF2(-/-) mice on both the C57BL/6J and FVB/NJ backgrounds exhibited mPIN lesions as previously reported on a 129P2/OLA-C57BL/6J background. In contrast to the 129P2/OLA-C57BL/6J mixed genetic background, the mPIN lesions in C57BL/6J and FVB/NJ EAF2(-/-) mice were associated with stromal defects characteristic of a reactive stroma and a statistically significant increase in prostate microvessel density. Stromal inflammation and increased microvessel density was evident in EAF2-deficient mice on a pure C57BL/6J background at an early age and preceded the development of the histologic epithelial hyperplasia and neoplasia found in the prostates of older EAF2(-/-) animals. Mice deficient in EAF2 had an increased recovery rate and a decreased overall response to the effects of androgen deprivation. EAF2 expression in human cancer was significantly down-regulated and microvessel density was significantly increased compared to matched normal prostate tissue; furthermore EAF2 expression was negatively correlated with microvessel density. These results suggest that the EAF2 knockout mouse on the C57BL/6J and FVB/NJ genetic backgrounds provides a model of PIN lesions associated with an altered prostate microvasculature and reactive stromal compartment corresponding to that reported in human prostate tumors. | | | 24260246
 |
Cyp1b1 mediates periostin regulation of trabecular meshwork development by suppression of oxidative stress.
Zhao, Y; Wang, S; Sorenson, CM; Teixeira, L; Dubielzig, RR; Peters, DM; Conway, SJ; Jefcoate, CR; Sheibani, N
Molecular and cellular biology
33
4225-40
2013
Show Abstract
Mutation in CYP1B1 has been reported for patients with congenital glaucoma. However, the underlying mechanisms remain unknown. Here we show increased diurnal intraocular pressure (IOP) in Cyp1b1-deficient (Cyp1b1(-/-)) mice. Cyp1b1(-/-) mice presented ultrastructural irregular collagen distribution in their trabecular meshwork (TM) tissue along with increased oxidative stress and decreased levels of periostin (Postn). Increased levels of oxidative stress and decreased levels of Postn were also detected in human glaucomatous TM tissues. Furthermore, Postn-deficient mice exhibited TM tissue ultrastructural abnormalities similar to those of Cyp1b1(-/-) mice. Administration of the antioxidant N-acetylcysteine (NAC) restored structural abnormality of TM tissue in Cyp1b1(-/-) mice. In addition, TM cells prepared from Cyp1b1(-/-) mice exhibited increased oxidative stress, altered adhesion, and decreased levels of Postn. These aberrant cellular responses were reversed in the presence of NAC or by restoration of Cyp1b1 expression. Cyp1b1 knockdown or inhibition of CYP1B1 activity in Cyp1b1(+/+) TM cells resulted in a Cyp1b1(-/-) phenotype. Thus, metabolic activity of CYP1B1 contributes to oxidative homeostasis and ultrastructural organization and function of TM tissue through modulation of Postn expression. | Dot Blot | Mouse | 23979599
 |
Aberrant production of extracellular matrix proteins and dysfunction in kidney endothelial cells with a short duration of diabetes.
Grutzmacher, C; Park, S; Zhao, Y; Morrison, ME; Sheibani, N; Sorenson, CM
American journal of physiology. Renal physiology
304
F19-30
2013
Show Abstract
Diabetic nephropathy is the most common cause of end-stage renal disease and is a major risk factor for cardiovascular disease. In the United States, microvascular complications during diabetic nephropathy contribute to high morbidity and mortality rates. However, the cell-autonomous impact of diabetes on kidney endothelial cell function requires further investigation. Male Akita/+ [autosomal dominant mutation in the insulin II gene (Ins2)] mice reproducibly develop diabetes by 4 wk of age. Here, we examined the impact a short duration of diabetes had on kidney endothelial cell function. Kidney endothelial cells were prepared from nondiabetic and diabetic mice (4 wk of diabetes) to delineate the early changes in endothelial cell function. Kidney endothelial cells from Akita/+ mice following 4 wk of diabetes demonstrated aberrant expression of extracellular matrix proteins including decreased osteopontin and increased fibronectin expression which correlated with increased α5-integrin expression. These changes were associated with the attenuation of migration and capillary morphogenesis. Kidney endothelial cells from Akita/+ mice had decreased VEGF levels but increased levels of endothelial nitric oxide synthase(eNOS) and NO, suggesting uncoupling of VEGF-mediated NO production. Knocking down eNOS expression in Akita/+ kidney endothelial cells increased VEGF expression, endothelial cell migration, and capillary morphogenesis. Furthermore, attenuation of sprouting angiogenesis of aortas from Akita/+ mice with 8 wk of diabetes was restored in the presence of the antioxidant N-acetylcysteine. These studies demonstrate that aberrant endothelial cell function with a short duration of diabetes may set the stage for vascular dysfunction and rarefaction at later stages of diabetes. | Immunohistochemistry | Rat | 23077100
 |
Lack of Cyp1b1 promotes the proliferative and migratory phenotype of perivascular supporting cells.
Palenski, TL; Sorenson, CM; Jefcoate, CR; Sheibani, N
Laboratory investigation; a journal of technical methods and pathology
93
646-62
2013
Show Abstract
Perivascular supporting cells, including pericytes and smooth muscle cells (PC/SMC), have an integral role during angiogenesis and control vascular remodeling, maturation, and stabilization of neoteric vessels. We recently showed that a Cyp1B1 deficiency in mice results in the attenuation of angiogenesis in vivo and the pro-angiogenic activity of endothelial cells in vitro. However, the contribution of PC/SMC, and more specifically the cell autonomous effects of Cyp1B1 in these processes, needs further investigation. Here we demonstrate that PC constitutively expressed Cyp1B1, and that a deficiency in Cyp1B1 was associated with enhanced proliferation, and decreased apoptosis. Mechanistically, the lack of Cyp1B1 was associated with increased oxidative stress and sustained NF-κB activation, which was reversed by the antioxidant N-acetylcysteine. These changes were also concomitant with alterations in PC migration, adhesion, and expression of various extracellular matrix proteins, including thrombospondin-2. Cyp1B1-deficient PC also expressed decreased levels of vascular endothelial growth factor. Together, our results suggest an important role for Cyp1B1 expression in the regulation of PC proliferation, migration, and survival through modulation of the intracellular oxidative state and NF-κB expression and/or activity. Thus, a lack of Cyp1B1 in PC may have a significant role in vascular dysfunction and integrity, contributing to the attenuation of angiogenesis. | Immunoblotting (Western) | Mouse | 23568032
 |
Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration.
Calve, S; Simon, HG
FASEB journal : official publication of the Federation of American Societies for Experimental Biology
26
2538-45
2012
Show Abstract
During forelimb regeneration in the newt Notophthalmus viridescens, the dynamic expression of a transitional matrix rich in hyaluronic acid, tenascin-C, and fibronectin controls muscle cell behavior in vivo and in vitro. However, the influence of extracellular matrix (ECM) remodeling on tissue stiffness and the cellular response to mechanical variations during regeneration was unknown. By measuring the transverse stiffness of tissues in situ, we found undifferentiated regenerative blastemas were less stiff than differentiated stump muscle (13.3±1.6 vs. 16.6±1.2 kPa). To directly determine how ECM and stiffness combine to affect skeletal muscle fragmentation, migration, and fusion, we coated silicone-based substrates ranging from 2 to 100 kPa with matrices representative of transitional (tenascin-C and fibronectin) and differentiated environments (laminin and Matrigel). Using live-cell imaging, we found softer tenascin-C-coated substrates significantly enhanced migration and fragmentation of primary newt muscle cells. In contrast, stiffer substrates coated with laminin, Matrigel, or fibronectin increased differentiation while suppressing migration and fragmentation. These data support our in vivo observations that a transitional matrix of reduced stiffness regulates muscle plasticity and progenitor cell recruitment into the regenerative blastema. These new findings will enable the determination of how biochemical and mechanical cues from the ECM control genetic pathways that drive regeneration. | Immunohistochemistry | | 22415307
 |
BIM deficiency differentially impacts the function of kidney endothelial and epithelial cells through modulation of their local microenvironment.
Sheibani, N; Morrison, ME; Gurel, Z; Park, S; Sorenson, CM
American journal of physiology. Renal physiology
302
F809-19
2012
Show Abstract
The extracellular matrix (ECM) acts as a scaffold for kidney cellular organization. Local secretion of the ECM allows kidney cells to readily adapt to changes occurring within the kidney. In addition to providing structural support for cells, the ECM also modulates cell survival, migration, proliferation, and differentiation. Although aberrant regulation of ECM proteins can play a causative role in many diseases, it is not known whether ECM production, cell adhesion, and migration are regulated in a similar manner in kidney epithelial and endothelial cells. Here, we demonstrate that lack of BIM expression differentially impacts kidney endothelial and epithelial cell ECM production, migration, and adhesion, further emphasizing the specialized role of these cell types in kidney function. Bim -/- kidney epithelial cells demonstrated decreased migration, increased adhesion, and sustained expression of osteopontin and thrombospondin-1 (TSP1). In contrast, bim -/- kidney endothelial cells demonstrated increased cell migration, and decreased expression of osteopontin and TSP1. We also observed a fivefold increase in VEGF expression in bim -/- kidney endothelial cells consistent with their increased migration and capillary morphogenesis. These cells also had decreased endothelial nitric oxide synthase activity and nitric oxide bioavailability. Thus kidney endothelial and epithelial cells make unique contributions to the regulation of their ECM composition, with specific impact on adhesive and migratory properties that are essential for their proper function. | Immunofluorescence | Mouse | 22169007
 |