Review: The role of leukaemia inhibitory factor in the establishment of pregnancy.
Vogiagis, D and Salamonsen, L A
J. Endocrinol., 160: 181-90 (1999)
1999
Show Abstract
Leukaemia inhibitory factor (LIF) is a pleiotrophic cytokine required for blastocyst implantation in mice. Uterine expression of LIF and that of its receptors has been demonstrated in a number of mammalian species indicating that LIF may have widespread importance in the establishment of pregnancy. The variations in the reaction of the uterus in preparation for and during implantation are considerable between species and understanding the differences and similarities assists in the interpretation of how this cytokine functions. Recent studies suggest that reduced endometrial LIF contributes to human infertility. Studies also demonstrate a potential role in placentation and fetal development. Thus, LIF has become an important cytokine warranting further investigation in the human. It is anticipated that when the mechanisms underlying normal embryonic and endometrial development are elucidated, fertility and infertility will be more precisely understood and hence able to be effectively controlled. | 9924186
 |
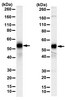
In vivo and in vitro regulation of thyroid leukemia inhibitory factor (LIF): marker of hypothyroidism.
Ren, S G, et al.
J. Clin. Endocrinol. Metab., 84: 2883-7 (1999)
1999
Show Abstract
Several cytokines regulate thyroid function and may be involved in the pathogenesis of thyroid disorders, including euthyroid sick syndrome. Leukemia inhibitory factor (LIF), a neuroimmune pleiotropic cytokine, was measured to assess its role in hypothalamic-pituitary-thyroid function. Mean circulating serum LIF levels in 10 hypothyroid patients [TSH, 23+/-0.5 mIU/L (mean+/-SEM); free T4, 0.77+/-0.1 ng/dL] was 0.29+/-0.04 ng/mL, 145% higher (P < 0.04) than in 20 normal subjects (LIF, 0.20+/-0.02 ng/mL; TSH, 2.23+/-0.21 mIU/L; free T4, 1.23+/-0.04 ng/dL) but was not different from those in 10 hyperthyroid patients (LIF, 0.21+/-0.03 ng/mL; TSH, 0.01+/-0.00 mIU/L; free T4, 3.63+/-0.51 ng/dL). Serum LIF concentrations linearly correlated with serum TSH in the 40 samples (r = 0.58, P < 0.001). When T4 (1-8 microg/kg x day) was administered to cynomolgus monkeys with methimazole-induced hypothyroidism, serum T4 and T3 levels increased appropriately, and TSH and LIF concentrations decreased. When methimazole was given alone, both serum TSH (146+/-30 mIU/L) and LIF (8.84+/-0.49 ng/mL) were markedly induced. When methimazole together with T4 (>2 microg/kg x day) was administered, both serum TSH (7.5+/-1.2 mIU/L) and LIF (6.22+/-0.31 ng/mL) were lowered (P < 0.01). Monkey serum LIF levels and log TSH levels also correlated (r = 0.72, P < 0.01). Cultured thyroid carcinoma cells produced LIF (9.2 ng/10(6) cells/48 h). TSH (100 mIU/mL) and interleukin (IL)-6 (10 nmol/L) stimulated in vitro LIF secretion from the cells by 170+/-12% (P < 0.05) and 261+/-8% (P < 0.05), respectively. Dexamethasone (1 micromol/L) inhibited basal LIF concentration by 83% (P < 0.05), whereas TSH and IL-6 stimulated LIF by 52% (P = 0.04) and 42% (P = 0.03), respectively. However, using Northern blot analysis, we could not observe induction of LIF mRNA by TSH, suggesting that LIF regulation by TSH may be due to stimulation of secretion. The results show that the thyroid gland is a source of LIF production; TSH, IL-6, and glucocorticoid influence thyroid cell LIF expression. The correlation between TSH and LIF suggests that LIF may participate in the physiologic regulation of hypothalamic-pituitary-thyroid function. | 10443695
 |
Leukemia inhibitory factor, glial cell line-derived neurotrophic factor, and their receptor expressions following muscle crush injury.
Kami, K, et al.
Muscle Nerve, 22: 1576-86 (1999)
1999
Show Abstract
Using in situ hybridization histochemistry, we characterized the spatiotemporal gene expression patterns of leukemia inhibitory factor (LIF) and glial cell line-derived neurotrophic factor (GDNF), and their receptor components (LIFR, GFR-alpha1, RET) induced in muscle cells, intramuscular nerves, and motoneurons in the regeneration processes of both muscle cells and nerves following muscle contusion. Muscle contusion induced upregulation of GDNF and GFR-alpha1 mRNAs in Schwann cell-like cells in the intramuscular nerves and of LIFR mRNA in damaged muscle cells. LIFR, GFR-alpha1, and RET mRNA expressions in motoneurons were upregulated following muscle contusion. Muscle contusion also induced more rapid, prominent transactivations of GFR-alpha1 and RET genes in motoneurons than did sciatic nerve axotomy. These findings suggest that rapid and prominent upregulation of the receptor components for LIF and GDNF in motoneurons is important for the regeneration of intramuscular motor nerves damaged by muscle contusion. | 10514237
 |
In vitro expansion of a multipotent population of human neural progenitor cells.
Carpenter, M K, et al.
Exp. Neurol., 158: 265-78 (1999)
1999
Show Abstract
The isolation and expansion of human neural progenitor cells have important potential clinical applications, because these cells may be used as graft material in cell therapies to regenerate tissue and/or function in patients with central nervous system (CNS) disorders. This paper describes a continuously dividing multipotent population of progenitor cells in the human embryonic forebrain that can be propagated in vitro. These cells can be maintained and expanded using a serum-free defined medium containing basic fibroblast growth factor (bFGF), leukemia inhibitory factor (LIF), and epidermal growth factor (EGF). Using these three factors, the cell cultures expand and remain multipotent for at least 1 year in vitro. This period of expansion results in a 10(7)-fold increase of this heterogeneous population of cells. Upon differentiation, they form neurons, astrocytes, and oligodendrocytes, the three main phenotypes in the CNS. Moreover, GABA-immunoreactive and tyrosine hydroxylase-immunoreactive neurons can be identified. These results demonstrate the feasibility of long-term in vitro expansion of human neural progenitor cells. The advantages of such a population of neural precursors for allogeneic transplantation include the ability to provide an expandable, well-characterized, defined cell source which can form specific neuronal or glial subtypes. | 10415135
 |