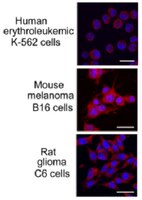
Exogenous protein Hsp70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures.
Ekimova, IV; Nitsinskaya, LE; Romanova, IV; Pastukhov, YF; Margulis, BA; Guzhova, IV
Journal of neurochemistry
115
1035-44
2010
Show Abstract
Heat shock protein 70 kDa (Hsp70) possesses a remarkable neuroprotective activity and the results of recent studies demonstrated its efficacy in the attenuation of epileptic seizures. The aim of this study was to explore the effects of a pure Hsp70/Hsc70 preparation delivered to the brain regions involved in generalized seizures induced in rats by intracerebroventricular microinjections of NMDA or systemic injections of pentylenetetrazole. Purified Hsp70/Hsc70 was administered (intracerebroventricular) 2 h before the induction of seizures. Compared to the vehicle-treated control animals, Hsp70/Hsc70-pretreated rats demonstrated reduced severity of NMDA- and pentylenetetrazole-induced seizures. To identify the brain structures potentially implicated in the Hsp70/Hsc70-mediated anticonvulsant effect, we analysed the localization of a fluorescently-labelled chaperone in the brain. Labelled Hsp70/Hsc70 was found in neurons and terminals of the limbic seizure complex of the brain and was co-localized in these regions with NMDA receptors, synaptophysin and the GABA-synthesizing enzyme, L-glutamic acid decarboxylase 67. An immunoprecipitation assay confirmed interactions between Hsp70 and both synaptophysin and L-glutamic acid decarboxylase 67 in brain tissue. We suggest that the anticonvulsant effect of exogenous Hsp70/Hsc70 is not only based on its protective capacity but is also related to its ability to modulate GABA neurotransmission, which in turn contributes to the maintenance of the excitatory-inhibitory balance of the CNS. | 20831598
 |
Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death.
Høyer-Hansen, M; Bastholm, L; Mathiasen, IS; Elling, F; Jäättelä, M
Cell death and differentiation
12
1297-309
2005
Show Abstract
A chemotherapeutic vitamin D analogue, EB1089, kills tumor cells via a caspase-independent pathway that results in chromatin condensation and DNA fragmentation. Employing transmission- and immunoelectronmicroscopy as well as detection of autophagosome-associated LC3-beta protein in the vacuolar structures, we show here that EB1089 also induces massive autophagy in MCF-7 cells. Interestingly, inhibition of autophagy effectively hindered apoptosis-like nuclear changes and cell death in response to EB1089. Furthermore, restoration of normal levels of beclin 1, an autophagy-inducing tumor suppressor gene that is monoallelically deleted in MCF-7 cells, greatly enhanced the EB1089-induced nuclear changes and cell death. Thus, EB1089 triggers nuclear apoptosis via a pathway involving Beclin 1-dependent autophagy. Surprisingly, tumor cells depleted for Beclin 1 failed to proliferate suggesting that even though the monoallelic depletion of beclin 1 in human cancer cells suppresses EB1089-induced autophagic death, one intact beclin 1 allele is essential for tumor cell proliferation. | 15905882
 |
Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2.
Nylandsted, J; Rohde, M; Brand, K; Bastholm, L; Elling, F; Jäättelä, M
Proceedings of the National Academy of Sciences of the United States of America
97
7871-6
2000
Show Abstract
Heat shock protein 70 is an antiapoptotic chaperone protein highly expressed in human breast tumors and tumor cell lines. Here, we demonstrate that the mere inhibition of its synthesis by adenoviral transfer or classical transfection of antisense Hsp70 cDNA (asHsp70) results in massive death of human breast cancer cells (MDA-MB-468, MCF-7, BT-549, and SK-BR-3), whereas the survival of nontumorigenic breast epithelial cells (HBL-100) or fibroblasts (WI-38) is not affected. Despite the apoptotic morphology as judged by electron microscopy, the asHsp70-induced death was independent of known caspases and the p53 tumor suppressor protein. Furthermore, Bcl-2 and Bcl-X(L), which protect tumor cells from most forms of apoptosis, failed to rescue breast cancer cells from asHsp70-induced death. These results show that tumorigenic breast cancer cells depend on the constitutive high expression of Hsp70 to suppress a transformation-associated death program. Neutralization of Hsp70 may open new possibilities for treatment of cancers that have acquired resistance to therapies activating the classical apoptosis pathway. | 10884417
 |
Heat shock proteins in cardiosurgery patients.
Demidov, ON; Tyrenko, VV; Svistov, AS; Komarova, YY; Karpishenko, AI; Margulis, BA; Shevchenko, YL
European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery
16
444-9
1999
Show Abstract
Cytoplasmic members of the heat shock protein HSP70, family, inducible HSP72 and constitutive HSC73, are known to protect cells and organisms against harmful factors including ischemia, trauma, etc. The up-regulation of HSP70 was shown to greatly increase resistance of myocardial cells in vitro as well as in transgenic animals. It seems reasonable to expect that in patients undergoing open heart surgery cytoplasmic HSP70 should play a protective role, reducing the risk of the myocardial cell injury.Using Western blotting, we determined levels of HSP72 and HSC73 in myocardium and peripheral blood lymphocytes of 51 patients with coronary and valvular diseases. In all the cases, HSP70 was detected in samples of the right atria before and after cardiopulmonary bypass.Induction of HSP72 was observed in 40% of all patients and correlated with the endurance of cardiopulmonary bypass and with disease duration in 33 patients with coronary artery disease. The cardioprotective effect of the elevated pre-operational level of HSP72 was shown to correlate with the lower activity of cardiospecific enzymes in the coronary disease patients. The HSC73 level in the right atria did not depend on conditions of the open heart surgery, while in some cases, it was increased after bypass. No correlation has been found between preoperational content of HSP72/HSC73 in lymphocytes and its pre- or post-bypass content in myocardium.HSP72 is implicated in cardioprotection in combination with some other factors, and its pre-operational level, among other parameters, might be of prognostic value. | 10571093
 |