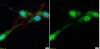
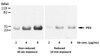
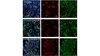
AM34 Sigma-AldrichAnti-Caspase-3 (Ab-2) Mouse mAb (10C1.C9)
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Species Reactivity | Host | Antibody Type |
|---|---|---|
| H | M | Monoclonal Antibody |
| Product Information | |
|---|---|
| Form | Liquid |
| Formulation | In 0.05 M sodium phosphate buffer, 0.2% gelatin, pH 7.4. |
| Positive control | Jurkat cells |
| Preservative | ≤0.1% sodium azide |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Storage and Shipping Information | |
|---|---|
| Ship Code | Blue Ice Only |
| Toxicity | Standard Handling |
| Storage | +2°C to +8°C |
| Do not freeze | Yes |
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| AM34 | 0 |
Documentation
Anti-Caspase-3 (Ab-2) Mouse mAb (10C1.C9) SDS
| Title |
|---|
Anti-Caspase-3 (Ab-2) Mouse mAb (10C1.C9) Certificates of Analysis
| Title | Lot Number |
|---|---|
| AM34 |
References
| Reference overview |
|---|
| Alnemri, E.S. 1997. J. Cell. Biochem. 64, 33. Bayaert, R., et al. 1997. J. Biol. Chem. 272, 11694. Erhardt, P., et al. 1997. J. Biol. Chem. 272, 15049. Inayat-Hussain, S.H., et al. 1997. Hepatology 25, 1516. Krjewska, M., et al. 1997. Cancer Res. 57, 1605. Mashima, T., et al. 1997. Oncogene 14, 1007. McConnell, K.R., et al. 1997. J. Immunol. 158, 2083. Posmantur, R., et al. 1997. J. Neurochem. 68, 2328. Suzuki, A., et al. 1997. Exp. Cell. Res. 233, 48. Liu, X., et al. 1996. J. Biol. Chem. 271, 13371. Quan, L.T., et al. 1996. Proc. Natl. Acad. Sci. USA 93, 1972. Teraoka, H., et al. 1996. FEBS Lett. 393, 1. Tiso, N., et al. 1996. Biochem. Biophys. Res. Commun. 225 983. Xue, D., et al. 1996. Genes Dev. 10, 1073. Tewari, M., et al. 1995. Cell 81, 801. Fernandes-Alnemri, T., et al. 1994. J. Biol. Chem. 269, 30761. |
Brochure
| Title |
|---|
| Caspases and other Apoptosis Related Tools Brochure |