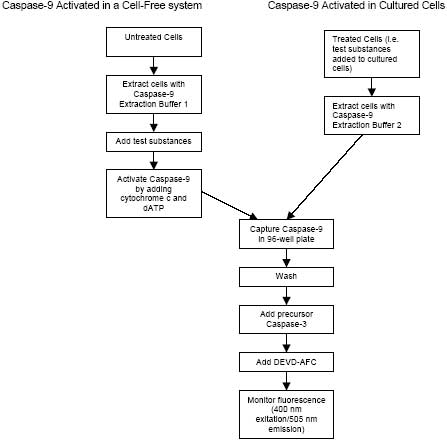
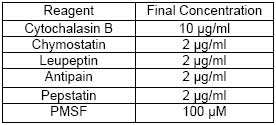
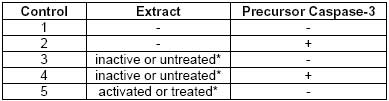
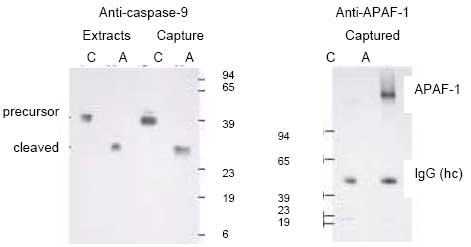
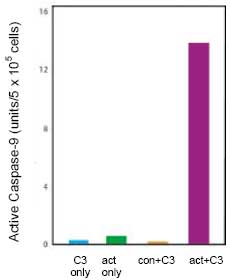
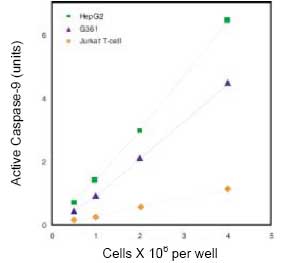
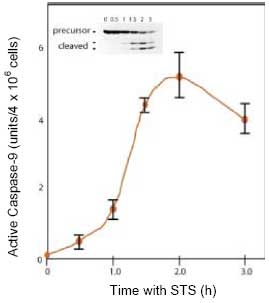
CBA047 Active Caspase-9 Assay Kit
Recommended Products
Overview
| Replacement Information |
|---|
| References |
|---|
| Product Information | |
|---|---|
| Detection method | Fluorometric |
| Form | 480 Tests |
| Format | 96-well plate |
| Kit contains | Caspase-9 Capture Antibody, Precursor Caspase-3, DEVD-AFC, and a user protocol. |
| Applications |
|---|
| Biological Information | |
|---|---|
| Sample Type | Cells |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Storage and Shipping Information | |
|---|---|
| Ship Code | Blue Ice Only |
| Toxicity | Standard Handling |
| Storage | -20°C |
| Do not freeze | Ok to freeze |
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information | |
|---|---|
| Kit contains | Caspase-9 Capture Antibody, Precursor Caspase-3, DEVD-AFC, and a user protocol. |
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| CBA047 | 0 |
Documentation
Brochure
| Title |
|---|
| Kit SourceBook - 2nd Edition EURO |
| Kits SourceBook - 2nd Edition GBP |