534034 Sigma-AldrichHeterochromatin Inhibitor, HMS-I1 - Calbiochem
A cell-permeable, reversible inhibitor of heterochromatin-mediated gene silencing in mammalian, plant, and fungal cells (5 - 10 µM).
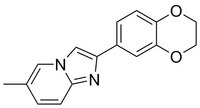
More>> A cell-permeable, reversible inhibitor of heterochromatin-mediated gene silencing in mammalian, plant, and fungal cells (5 - 10 µM). Less<<Synonyms: 2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-6-methylimidazo[1,2-a]pyridine, 2-(2,3-Dihydro-1,4-benzo[b][1,4]dioxin-6-yl)-6-methylimidazo[1,2-a]pyridine, Heterochromatin Mediated Silencing Inhibitor 1
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₁₆H₁₄N₂O₂ |
| References | |
|---|---|
| References | Castonguay, E., et al. 2015. Mol. Cell. Biol. 35, 662. |
| Product Information | |
|---|---|
| Form | Pale yellow solid |
| Hill Formula | C₁₆H₁₄N₂O₂ |
| Chemical formula | C₁₆H₁₄N₂O₂ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Clr3-containing Snf2/HDAC repressor complex (SHREC) |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 534034 | 0 |
Documentation
Heterochromatin Inhibitor, HMS-I1 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Castonguay, E., et al. 2015. Mol. Cell. Biol. 35, 662. |