538337 Sigma-AldrichPDE9 Inhibitor, PF-04447943 - CAS 1082744-20-4 - Calbiochem
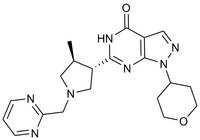
Synonyms: 6-((3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl)-1-(tetrahydro-2H-pyran-4-yl)-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-one, PF04447943, PF 04447943, Phosphodiesterase 9A Inhibitor, PF-04447943
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 1082744-20-4 | C₂₀H₂₅N₇O₂ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 5.38337.0001 |
|
Botol kaca | 10 mg |
|
— |
| References | |
|---|---|
| References | Lee, D., et al. 2015. Nature. 519, 472 Verhoest, P. R., et al. 2012. J. Med. Chem. 55, 9045. Hutson, P.H., et al. 2011. Neuropharmacology. 61, 665. |
| Product Information | |
|---|---|
| CAS number | 1082744-20-4 |
| Form | Off-white solid |
| Hill Formula | C₂₀H₂₅N₇O₂ |
| Chemical formula | C₂₀H₂₅N₇O₂ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | PDE9 |
| Primary Target IC<sub>50</sub> | 2.8 nM, 4.5 nM and 18 nM for human, rhesus and rat PDE9A inhibition respectively |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.38337.0001 | 04054839059438 |
Documentation
PDE9 Inhibitor, PF-04447943 - CAS 1082744-20-4 - Calbiochem MSDS
| Title |
|---|
References
| Reference overview |
|---|
| Lee, D., et al. 2015. Nature. 519, 472 Verhoest, P. R., et al. 2012. J. Med. Chem. 55, 9045. Hutson, P.H., et al. 2011. Neuropharmacology. 61, 665. |