488250 MilliporeNon-Interfering Protein Assay™ Kit
Accurate protein quantification from solutions containing interfering compounds
More>> Accurate protein quantification from solutions containing interfering compounds Less<<Recommended Products
Overview
| Replacement Information |
|---|
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 488250-1KTT |
|
Botol kaca | 1 ktt |
|
— |
| Applications |
|---|
| Biological Information |
|---|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information | |
|---|---|
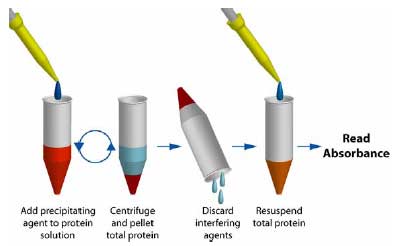
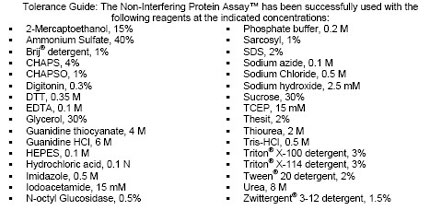
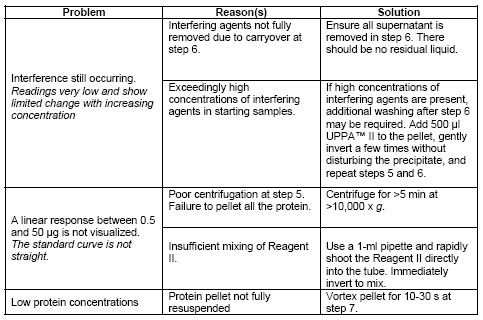
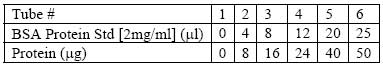
| Kit contains | UPPA™ Reagents I and II, Copper Solution I, Color Agents A and B, BSA Standard, and a user protocol. |
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 488250-1KTT | 04055977200478 |
Documentation
Non-Interfering Protein Assay™ Kit Certificates of Analysis
| Title | Lot Number |
|---|---|
| 488250 |
References
| Reference overview |
|---|
| Ho, T.H. et al. 2005. Human Molecular Genetics 14, 1539. Ladd, A.N. et al. 2001. Mol. Cell. Biol. 21, 1285. Loeb, D.M. et al. 2002. J. Biol. Chem. 277, 19627. Shiels, A. et al. 2007. Invest. Ophthalmol. Vis. Sci. 48, 500. Gushwa, N.N. et al. 2003. Plant Physiology 132, 1925. DePinto, W. et al. 2006. Mol. Cancer Ther. 5, 2644 Werner, M.E. et al. 2007. J. Biol. Chem. 282, 5560. Dennison, S.M. et al (2006). Biophysical Journal 90, 1661. Reeve, I. et al. 2002. PNAS 99, 8608. |