239806 Sigma-AldrichInSolution™ Cyclopamine, V. californicum - Calbiochem
The InSolution™ Cyclopamine, V. californicum controls the biological activity of Sonic Hedgehog signaling pathway. This small molecule/inhibitor is primarily used for Cancer applications.
More>> The InSolution™ Cyclopamine, V. californicum controls the biological activity of Sonic Hedgehog signaling pathway. This small molecule/inhibitor is primarily used for Cancer applications. Less<<Synonyms: Shh Signaling Antagonist I in EtOH
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₂₇H₄₁NO₂ |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 239806-5MG |
|
Botol kaca | 5 mg |
|
— |
| Description | |
|---|---|
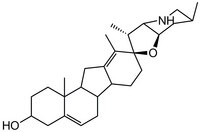
| Overview | A cell-permeable steroidal alkaloid and cholesterol mimic that displays both teratogenic and antitumor activities. It disrupts cholesterol bio-synthesis and specifically antagonizes shh (Sonic Hedgehog) signaling pathway through direct interaction with Smo (smoothened), a distant relative of G-protein-coupled receptors. A valuable tool for studying the involvement of shh signaling in the development of various tumors. Tomatidine (Cat. No. 614350) can be used as a negative control. The solid form of this compound (Cat. No. 239803) is also available. |
| Catalogue Number | 239806 |
| Brand Family | Calbiochem® |
| Synonyms | Shh Signaling Antagonist I in EtOH |
| Product Information | |
|---|---|
| Form | Liquid |
| Formulation | Supplied as a 10 mM (5 mg/1.22 mL) solution of Cyclopamine, V. californicum (Cat. No. 239803) in EtOH. |
| Hill Formula | C₂₇H₄₁NO₂ |
| Chemical formula | C₂₇H₄₁NO₂ |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥97% by NMR |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information | |
|---|---|
| R Phrase | R: 10 Flammable. |
| S Phrase | S: 16-7 Keep away from sources of ignition - No Smoking. Keep container tightly closed. |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 239806-5MG | 04055977198911 |
Documentation
InSolution™ Cyclopamine, V. californicum - Calbiochem MSDS
| Title |
|---|
InSolution™ Cyclopamine, V. californicum - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 239806 |
References
| Reference overview |
|---|
| Berman, D.M., et al. 2003. Nature, 425, 846. Thayer, S.P., et al. 2003. Nature, 425, 851. Watkins, D.N., et al. 2003. Nature 422, 313. Chen, J.K., et al. 2002. Proc. Natl. Acad. Sci. USA 99, 14071. Berman, D.M., et al. 2002. Science 297, 1559. Taipale, J., et al. 2000. Nature 406, 1005. Kim, S.K., and Melton, D.A. 1998. Proc. Natl. Acad. Sci. USA 95, 13036. Cooper, M.K., et al. 1998. Science 280, 1603. Incardona, J.P., et al. 1998. Development 125, 3553. |
| Data Sheet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|