375670 Sigma-AldrichHerbimycin A, Streptomyces sp. - CAS 70563-58-5 - Calbiochem
A potent and cell-permeable protein tyrosine kinase inhibitor.
More>> A potent and cell-permeable protein tyrosine kinase inhibitor. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
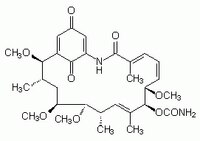
| 70563-58-5 | C₃₀H₄₂N₂O₉ |
| Product Information | |
|---|---|
| CAS number | 70563-58-5 |
| ATP Competitive | N |
| Form | Yellow solid |
| Hill Formula | C₃₀H₄₂N₂O₉ |
| Chemical formula | C₃₀H₄₂N₂O₉ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | P60v-src |
| Primary Target IC<sub>50</sub> | 12 µM against P60v-src |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | LX8930000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 375670 | 0 |
Documentation
Herbimycin A, Streptomyces sp. - CAS 70563-58-5 - Calbiochem MSDS
| Title |
|---|
Herbimycin A, Streptomyces sp. - CAS 70563-58-5 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 375670 |
References
| Reference overview |
|---|
| Fan, T.-P., et al. 1995. Trends Pharmacol. Sci. 16, 57. Kim, B.Y. 1995. Biochem. Biophys. Res. Commun. 212, 1061. Migita, K., et al. 1994. J. Immunol. 153, 3457. Okabe, M., et al. 1994. Leuk. Res. 18, 867. Fukazawa, H., et al. 1991. Biochem. Pharmacol. 42, 1661. Obinata, A., et al. 1991. Exp. Cell Res. 193, 36. Park, D.J., et al. 1991. J. Biol. Chem. 266, 24237. Weinstein, S.L., et al. 1991. Proc. Natl. Acad. Sci. USA 88, 4148. Oikawa, T., et al. 1989. Cancer Lett. 48, 157. Uehara, Y., et al. 1989. Biochem. Biophys. Res. Commun. 163, 803. Uehara, Y., et al. 1988. Virology 164, 294. Omura, S., et al. 1979. J. Antibiotics 32, 255. |
Citations
| Title | |
|---|---|
|
|