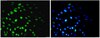
Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration.
Zhao, L; Zabel, MK; Wang, X; Ma, W; Shah, P; Fariss, RN; Qian, H; Parkhurst, CN; Gan, WB; Wong, WT
EMBO molecular medicine
7
1179-97
2015
Show Abstract
Retinitis pigmentosa, caused predominantly by mutations in photoreceptor genes, currently lacks comprehensive treatment. We discover that retinal microglia contribute non-cell autonomously to rod photoreceptor degeneration by primary phagocytosis of living rods. Using rd10 mice, we found that the initiation of rod degeneration is accompanied by early infiltration of microglia, upregulation of phagocytic molecules in microglia, and presentation of "eat-me" signals on mutated rods. On live-cell imaging, infiltrating microglia interact dynamically with photoreceptors via motile processes and engage in rapid phagocytic engulfment of non-apoptotic rods. Microglial contribution to rod demise is evidenced by morphological and functional amelioration of photoreceptor degeneration following genetic ablation of retinal microglia. Molecular inhibition of microglial phagocytosis using the vitronectin receptor antagonist cRGD also improved morphological and functional parameters of degeneration. Our findings highlight primary microglial phagocytosis as a contributing mechanism underlying cell death in retinitis pigmentosa and implicate microglia as a potential cellular target for therapy. | | | 26139610
 |
1D4: a versatile epitope tag for the purification and characterization of expressed membrane and soluble proteins.
Molday, LL; Molday, RS
Methods in molecular biology (Clifton, N.J.)
1177
1-15
2014
Show Abstract
Incorporation of short epitope tags into proteins for recognition by commercially available monoclonal or polyclonal antibodies has greatly facilitated the detection, characterization, localization, and purification of heterologously expressed proteins for structure-function studies. A number of tags have been developed, but many epitope-antibody combinations do not work effectively for all immunochemical techniques due to the nature of the tag and the specificity of the antibodies. A highly versatile, multipurpose epitope tag is the 9 amino acid C-terminal 1D4 peptide. This peptide tag together with the Rho1D4 monoclonal antibody can be used to detect proteins in complex mixtures by western blotting and ELISA assays, localize proteins in cells by immunofluorescence and immunoelectron microscopic labeling techniques, identify subunits and interacting proteins by co-immunoprecipitation, and purify functionally active proteins including membrane proteins by immunoaffinity chromatography. In this chapter we describe various immunochemical procedures which can be used for the detection, purification and localization of 1D4-tagged proteins for structure-function studies. | | | 24943310
 |
Changes in retinal morphology, electroretinogram and visual behavior after transient global ischemia in adult rats.
Zhao, Y; Yu, B; Xiang, YH; Han, XJ; Xu, Y; So, KF; Xu, AD; Ruan, YW
PloS one
8
e65555
2013
Show Abstract
The retina is a light-sensitive tissue of the central nervous system that is vulnerable to ischemia. The pathological mechanism underlying retinal ischemic injury is not fully understood. The purpose of this study was to investigate structural and functional changes of different types of rat retinal neurons and visual behavior following transient global ischemia. Retinal ischemia was induced using a 4-vessel occlusion model. Compared with the normal group, the number of βIII-tubulin positive retinal ganglion cells and calretinin positive amacrine cells were reduced from 6 h to 48 h following ischemia. The number of recoverin positive cone bipolar cells transiently decreased at 6 h and 12 h after ischemia. However, the fluorescence intensity of rhodopsin positive rod cells and fluorescent peanut agglutinin positive cone cells did not change after reperfusion. An electroretinogram recording showed that the a-wave, b-wave, oscillatory potentials and the photopic negative response were completely lost during ischemia. The amplitudes of the a- and b-waves were partially recovered at 1 h after ischemia, and returned to the control level at 48 h after reperfusion. However, the amplitudes of oscillatory potentials and the photopic negative response were still reduced at 48 h following reperfusion. Visual behavior detection showed there was no significant change in the time spent in the dark chamber between the control and 48 h group, but the distance moved, mean velocity in the black and white chambers and intercompartmental crosses were reduced at 48 h after ischemia. These results indicate that transient global ischemia induces dysfunction of retinal ganglion cells and amacrine cells at molecular and ERG levels. However, transient global ischemia in a 17 minute duration does not appear to affect photoreceptors. | | | 23776500
 |
Involvement of NT3 and P75(NTR) in photoreceptor degeneration following selective Müller cell ablation.
Shen, W; Zhu, L; Lee, SR; Chung, SH; Gillies, MC
Journal of neuroinflammation
10
137
2013
Show Abstract
Neurotrophins can regulate opposing functions that result in cell survival or apoptosis, depending on which form of the protein is secreted and which receptor and signaling pathway is activated. We have recently developed a transgenic model in which inducible and patchy Müller cell ablation leads to photoreceptor degeneration. This study aimed to examine the roles of mature neurotrophin-3 (NT3), pro-NT3 and p75 neurotrophin receptor (P75(NTR)) in photoreceptor degeneration in this model.Transgenic mice received tamoxifen to induce Müller cell ablation. Changes in the status of Müller and microglia cells as well as expression of mature NT3, pro-NT3 and P75(NTR) were examined by immunohistochemistry and Western blot analysis. Recombinant mature NT3 and an antibody neutralizing 75(NTR) were injected intravitreally 3 and 6 days after Müller cell ablation to examine their effects on photoreceptor degeneration and microglial activation.We found that patchy loss of Müller cells was associated with activation of surviving Müller cells and microglial cells, concurrently with reduced expression of mature NT3 and upregulation of pro-NT3 and P75(NTR). Intravitreal injection of mature NT3 and a neutralizing antibody to P75NTR, either alone or in combination, attenuated photoreceptor degeneration and the beneficial effect was associated with inhibition of microglial activation.Our data suggest that Müller cell ablation alters the balance between the protective and deleterious effects of mature NT3 and pro-NT3. Modulation of the neuroprotective action of mature NT3 and pro-apoptotic pro-NT3/P75(NTR) signaling may represent a novel pharmacological strategy for photoreceptor protection in retinal disease. | | | 24224958
 |
Interaction of complement factor h and fibulin3 in age-related macular degeneration.
Wyatt, MK; Tsai, JY; Mishra, S; Campos, M; Jaworski, C; Fariss, RN; Bernstein, SL; Wistow, G
PloS one
8
e68088
2013
Show Abstract
Age-related macular degeneration (AMD) is a major cause of vision loss. It is associated with development of characteristic plaque-like deposits (soft drusen) in Bruch's membrane basal to the retinal pigment epithelium (RPE). A sequence variant (Y402H) in short consensus repeat domain 7 (SCR7) of complement factor H (CFH) is associated with risk for "dry" AMD. We asked whether the eye-targeting of this disease might be related to specific interactions of CFH SCR7 with proteins expressed in the aging human RPE/choroid that could contribute to protein deposition in drusen. Yeast 2-hybrid (Y2H) screens of a retinal pigment epithelium/choroid library derived from aged donors using CFH SCR7 baits detected an interaction with EFEMP1/Fibulin 3 (Fib3), which is the locus for an inherited macular degeneration and also accumulates basal to macular RPE in AMD. The CFH/Fib3 interaction was validated by co-immunoprecipitation of native proteins. Quantitative Y2H and ELISA assays with different recombinant protein constructs both demonstrated higher affinity for Fib3 for the disease-related CFH 402H variant. Immuno-labeling revealed colocalization of CFH and Fib3 in globular deposits within cholesterol-rich domains in soft drusen in two AMD donors homozygous for CFH 402H (H/H). This pattern of labeling was quite distinct from those seen in examples of eyes with Y/Y and H/Y genotypes. The CFH 402H/Fib3 interaction could contribute to the development of pathological aggregates in soft drusen in some patients and as such might provide a target for therapeutic intervention in some forms of AMD. | | | 23840815
 |
Meckelin 3 is necessary for photoreceptor outer segment development in rat Meckel syndrome.
Tiwari, S; Hudson, S; Gattone, VH; Miller, C; Chernoff, EA; Belecky-Adams, TL
PloS one
8
e59306
2013
Show Abstract
Ciliopathies lead to multiorgan pathologies that include renal cysts, deafness, obesity and retinal degeneration. Retinal photoreceptors have connecting cilia joining the inner and outer segment that are responsible for transport of molecules to develop and maintain the outer segment process. The present study evaluated meckelin (MKS3) expression during outer segment genesis and determined the consequences of mutant meckelin on photoreceptor development and survival in Wistar polycystic kidney disease Wpk/Wpk rat using immunohistochemistry, analysis of cell death and electron microscopy. MKS3 was ubiquitously expressed throughout the retina at postnatal day 10 (P10) and P21. However, in the mature retina, MKS3 expression was restricted to photoreceptors and the retinal ganglion cell layer. At P10, both the wild type and homozygous Wpk mutant retina had all retinal cell types. In contrast, by P21, cells expressing rod- and cone-specific markers were fewer in number and expression of opsins appeared to be abnormally localized to the cell body. Cell death analyses were consistent with the disappearance of photoreceptor-specific markers and showed that the cells were undergoing caspase-dependent cell death. By electron microscopy, P10 photoreceptors showed rudimentary outer segments with an axoneme, but did not develop outer segment discs that were clearly present in the wild type counterpart. At p21 the mutant outer segments appeared much the same as the P10 mutant outer segments with only a short axoneme, while the wild-type controls had developed outer segments with many well-organized discs. We conclude that MKS3 is not important for formation of connecting cilium and rudimentary outer segments, but is critical for the maturation of outer segment processes. | | | 23516626
 |
A novel experimental mouse model of retinal detachment: complete functional and histologic recovery of the retina.
Zeng, R; Zhang, Y; Shi, F; Kong, F
Investigative ophthalmology & visual science
53
1685-95
2012
Show Abstract
To establish an experimental mouse model of retinal detachment (RD) created by corneal puncture (CP).Mouse corneas were punctured with a 30.5-gauge beveled needle, and the anterior chamber was penetrated. Histologic and functional changes of the retina were examined by light microscopy and electroretinography (ERG). Certain retinal cellular responses were examined by immunofluorescence microscopy. Internucleosomal DNA fragmentation in the retina was determined by terminal deoxynucleotidyl transferase-mediated uridine 5'-triphosphate-biotin nick-end labeling (TUNEL). RESULTS. CP caused transient leakage of aqueous humor along the needle shaft and immediate formation of multiple retinal blebs, which shrank and flattened within 24 hours. Bleb formation was associated with detachment of the neuroretina from the retinal pigment epithelium (RPE). After CP, the RPE cells underwent extensive transformation during retinal detachment/reattachment, but they resumed normal morphology on retinal reattachment around 10 to 13 days after CP. Relative to pre-CP ERG amplitudes, the punctured eyes showed decreases of 45% and 24% in scotopic and 7% and 12% in photopic b- and a-wave amplitudes, respectively, within 10 to 20 minutes after CP. The ERG amplitudes recovered fully by 12 hours after CP. No infiltrated cells were observed in the subretinal space, and no proliferating or TUNEL-positive cells were observed in the retina of the punctured eyes.Puncturing the mouse cornea can create transient RD, and the functional and histologic changes in the retina can subsequently recover. This experimental mouse model of RD mimics human traction and serous RD. | | | 22323470
 |
Functional and anatomical remodeling in human retinal detachment.
Clairton F de Souza,Michael Kalloniatis,Philip J Polkinghorne,Charles N J McGhee,Monica L Acosta
Experimental eye research
97
2012
Show Abstract
Rhegmatogenous retinal detachment is by far the most common indication for retinal surgery and a major cause of severe vision loss. Increased levels of glutamate found in the vitreous of human patients and persistent remodeling, even after reattachment, suggest substantial neurochemical, functional and anatomical changes have occurred in the detached retina. Therefore, this study was designed to characterize the morphological changes and glutamate receptor functionality in human rhegmatogenous retinal detachment. A cation channel permeating probe, agmatine (1-amino-4-guanidobutane; AGB), was employed to track endogenous and kainate (KA) driven channel functionality combined with immunocytochemical characterization of cellular remodeling. In the detached retina increased AGB permeability was identified in the outer retina while there was a decrease in the inner retina in basal conditions. KA receptors exhibited increased AGB permeability in ON bipolar cells and decreased permeability in calbindin labeled inner retinal cells. All retinal detachment samples demonstrated ectopic synaptic protein expression, photoreceptor processes extending toward the inner retina, and other remodeling features of retinal degeneration. These anatomical changes have been demonstrated in animal studies and are novel features unreported in primary cases of human retinal detachment. We conclude that deafferentation in retinal detachment leads to alteration of the glutamatergic pathway. | | | 22406310
 |
The 1D4 antibody labels outer segments of long double cone but not rod photoreceptors in zebrafish.
Yin, J; Brocher, J; Linder, B; Hirmer, A; Sundaramurthi, H; Fischer, U; Winkler, C
Investigative ophthalmology & visual science
53
4943-51
2012
Show Abstract
In experimental eye research, zebrafish has become a powerful model for human retina disorders. The purpose of the present study is the characterization of antibodies commonly employed in zebrafish models for rod photoreceptor degeneration.The 1D4 monoclonal antibody, developed against bovine rhodopsin, has been widely used in studies addressing structural and functional features of rhodopsin and was reported as an informative marker to stain rod outer segments in both mice and zebrafish. We have used transgenic reporter lines and histologic analysis to determine the photoreceptor types identified by 1D4 and other antibodies in zebrafish.We demonstrate that 1D4, in contrast to what has been reported previously, does not recognize rod outer segments in zebrafish, but instead labels long double cone outer segments consistent with sequence conservation of the respective epitope. As an alternative marker for zebrafish rods, we characterized the monoclonal antibody zpr-3, which was found to stain outer segments of both rods, as well as double cones.Our findings highlight the importance to confirm specificity of antibodies in cross-species experiments for correct interpretation of experimental data. Our findings clarify conflicting published information arising from studies using 1D4 and zpr-3 antibodies in zebrafish. | | Zebrafish | 22743318
 |
Selective loss of RPGRIP1-dependent ciliary targeting of NPHP4, RPGR and SDCCAG8 underlies the degeneration of photoreceptor neurons.
Patil, H, et al.
Cell Death Dis, 3: e355 (2012)
2012
Show Abstract
The retinitis pigmentosa GTPase regulator (RPGR) and nephrocystin-4 (NPHP4) comprise two key partners of the assembly complex of the RPGR-interacting protein 1 (RPGRIP1). Mutations in RPGR and NPHP4 are linked to severe multisystemic diseases with strong retinal involvement of photoreceptor neurons, whereas those in RPGRIP1 cause the fulminant photoreceptor dystrophy, Leber congenital amaurosis (LCA). Further, mutations in Rpgrip1 and Nphp4 suppress the elaboration of the outer segment compartment of photoreceptor neurons by elusive mechanisms, the understanding of which has critical implications in uncovering the pathogenesis of syndromic retinal dystrophies. Here we show RPGRIP1 localizes to the photoreceptor connecting cilium (CC) distally to the centriole/basal body marker, centrin-2 and the ciliary marker, acetylated-α-tubulin. NPHP4 abuts proximally RPGRIP1, RPGR and the serologically defined colon cancer antigen-8 (SDCCAG8), a protein thought to partake in the RPGRIP1 interactome and implicated also in retinal-renal ciliopathies. Ultrastructurally, RPGRIP1 localizes exclusively throughout the photoreceptor CC and Rpgrip1(nmf247) photoreceptors present shorter cilia with a ruffled membrane. Strikingly, Rpgrip1(nmf247) mice without RPGRIP1 expression lack NPHP4 and RPGR in photoreceptor cilia, whereas the SDCCAG8 and acetylated-α-tubulin ciliary localizations are strongly decreased, even though the NPHP4 and SDCCAG8 expression levels are unaffected and those of acetylated-α-tubulin and γ-tubulin are upregulated. Further, RPGRIP1 loss in photoreceptors shifts the subcellular partitioning of SDCCAG8 and NPHP4 to the membrane fraction associated to the endoplasmic reticulum. Conversely, the ciliary localization of these proteins is unaffected in glomeruli or tubular kidney cells of Rpgrip1(nmf247), but NPHP4 is downregulated developmentally and selectively in kidney cortex. Hence, RPGRIP1 presents cell type-dependent pathological effects crucial to the ciliary targeting and subcellular partitioning of NPHP4, RPGR and SDCCAG8, and acetylation of ciliary α-tubulin or its ciliary targeting, selectively in photoreceptors, but not kidney cells, and these pathological effects underlie photoreceptor degeneration and LCA. | | | 22825473
 |