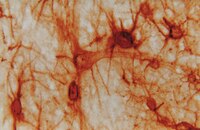
Abrasion arthroplasty increases mesenchymal stem cell content of postoperative joint effusions.
Beckmann, R; Lippross, S; Hartz, C; Tohidnezhad, M; Ferreira, MS; Neuss-Stein, S; Seekamp, A; Nebelung, S; Kweider, N; Rath, B; Jahr, H; Pufe, T; Varoga, DJ
BMC musculoskeletal disorders
16
250
2015
Show Abstract
Abrasion arthroplasty (AAP) is a procedure by which intrinsic cartilage healing is believed to be stimulated. Although clinically accepted for degenerative and traumatic cartilage lesions scientific evidence at a molecular level that proves the effect of AAP is scarce.Mononuclear cells were extracted from postoperative joint effusions 21.5 h post AAP and simple debridement of cartilage lesions. Luminex, ELISA and FACS experiments were performed. Immunohistochemical stainings of cell cultures for cartilage markers were used to confirm the findings.Postoperative joint effusions after AAP showed increased contents of Mononuclear cells compared to Arthroscopic Chondroplasty (ACP). BMP-4 and IGF were increased in AAP as complared to ACP. Mononuclear cells isolated after AAP express the MSC markers CD 73, CD 105, CD 90, CD 44 and are CD34 negative. Chondrogenic differentiation was demonstrated by positive staining for Sox9, collagen II, proteoglycan, chondroitin-4-sulfate.Our results support the clinical application of AAP as a procedure that enhances cartilage repair as an alternative to far more complex procedures that have gained popularity. Furthermore the data presented supports clinical investigations that recommend not to use suction drainage as by this procedure a considerable amount of the regeneratory potential of postoperative joint effusions might be extracted. | | | 26364138
 |
Local Delivery of High-Dose Chondroitinase ABC in the Sub-Acute Stage Promotes Axonal Outgrowth and Functional Recovery after Complete Spinal Cord Transection.
Cheng, CH; Lin, CT; Lee, MJ; Tsai, MJ; Huang, WH; Huang, MC; Lin, YL; Chen, CJ; Huang, WC; Cheng, H
PloS one
10
e0138705
2015
Show Abstract
Chondroitin sulfate proteoglycans (CSPGs) are glial scar-associated molecules considered axonal regeneration inhibitors and can be digested by chondroitinase ABC (ChABC) to promote axonal regeneration after spinal cord injury (SCI). We previously demonstrated that intrathecal delivery of low-dose ChABC (1 U) in the acute stage of SCI promoted axonal regrowth and functional recovery. In this study, high-dose ChABC (50 U) introduced via intrathecal delivery induced subarachnoid hemorrhage and death within 48 h. However, most SCI patients are treated in the sub-acute or chronic stages, when the dense glial scar has formed and is minimally digested by intrathecal delivery of ChABC at the injury site. The present study investigated whether intraparenchymal delivery of ChABC in the sub-acute stage of complete spinal cord transection would promote axonal outgrowth and improve functional recovery. We observed no functional recovery following the low-dose ChABC (1 U or 5 U) treatments. Furthermore, animals treated with high-dose ChABC (50 U or 100 U) showed decreased CSPGs levels. The extent and area of the lesion were also dramatically decreased after ChABC treatment. The outgrowth of the regenerating axons was significantly increased, and some partially crossed the lesion site in the ChABC-treated groups. In addition, retrograde Fluoro-Gold (FG) labeling showed that the outgrowing axons could cross the lesion site and reach several brain stem nuclei involved in sensory and motor functions. The Basso, Beattie and Bresnahan (BBB) open field locomotor scores revealed that the ChABC treatment significantly improved functional recovery compared to the control group at eight weeks after treatment. Our study demonstrates that high-dose ChABC treatment in the sub-acute stage of SCI effectively improves glial scar digestion by reducing the lesion size and increasing axonal regrowth to the related functional nuclei, which promotes locomotor recovery. Thus, our results will aid in the treatment of spinal cord injury. | | | 26393921
 |
Receptor protein tyrosine phosphatase σ binds to neurons in the adult mouse brain.
Yi, JH; Katagiri, Y; Yu, P; Lourie, J; Bangayan, NJ; Symes, AJ; Geller, HM
Experimental neurology
255
12-8
2014
Show Abstract
The role of type IIA receptor protein tyrosine phosphatases (RPTPs), which includes LAR, RPTPσ and RPTPδ, in the nervous system is becoming increasingly recognized. Evidence supports a significant role for these RPTPs during the development of the nervous system as well as after injury, and mutations in RPTPs are associated with human disease. However, a major open question is the nature of the ligands that interact with type IIA RPTPs in the adult brain. Candidates include several different proteins as well as the glycosaminoglycan chains of proteoglycans. In order to investigate this problem, we used a receptor affinity probe assay with RPTPσ-AP fusion proteins on sections of adult mouse brain and to cultured neurons. Our results demonstrate that the major binding sites for RPTPσ in adult mouse brain are on neurons and are not proteoglycan GAG chains, as RPTPσ binding overlaps with the neuronal marker NeuN and was not significantly altered by treatments which eliminate chondroitin sulfate, heparan sulfate, or both. We also demonstrate no overlap of binding of RPTPσ with perineuronal nets, and a unique modulation of RPTPσ binding to brain by divalent cations. Our data therefore point to neuronal proteins, rather than CSPGs, as being the ligands for RPTPσ in the adult, uninjured brain. | | | 24530640
 |
Reduced sulfation of chondroitin sulfate but not heparan sulfate in kidneys of diabetic db/db mice.
Reine, TM; Grøndahl, F; Jenssen, TG; Hadler-Olsen, E; Prydz, K; Kolset, SO
The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society
61
606-16
2013
Show Abstract
Heparan sulfate proteoglycans are hypothesized to contribute to the filtration barrier in kidney glomeruli and the glycocalyx of endothelial cells. To investigate potential changes in proteoglycans in diabetic kidney, we isolated glycosaminoglycans from kidney cortex from healthy db/+ and diabetic db/db mice. Disaccharide analysis of chondroitin sulfate revealed a significant decrease in the 4-O-sulfated disaccharides (D0a4) from 65% to 40%, whereas 6-O-sulfated disaccharides (D0a6) were reduced from 11% to 6%, with a corresponding increase in unsulfated disaccharides. In contrast, no structural differences were observed in heparan sulfate. Furthermore, no difference was found in the molar amount of glycosaminoglycans, or in the ratio of hyaluronan/heparan sulfate/chondroitin sulfate. Immunohistochemical staining for the heparan sulfate proteoglycan perlecan was similar in both types of material but reduced staining of 4-O-sulfated chondroitin and dermatan was observed in kidney sections from diabetic mice. In support of this, using qRT-PCR, a 53.5% decrease in the expression level of Chst-11 (chondroitin 4-O sulfotransferase) was demonstrated in diabetic kidney. These results suggest that changes in the sulfation of chondroitin need to be addressed in future studies on proteoglycans and kidney function in diabetes. | | | 23757342
 |
Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment.
Silver, DJ; Siebzehnrubl, FA; Schildts, MJ; Yachnis, AT; Smith, GM; Smith, AA; Scheffler, B; Reynolds, BA; Silver, J; Steindler, DA
The Journal of neuroscience : the official journal of the Society for Neuroscience
33
15603-17
2013
Show Abstract
Glioblastoma (GBM) remains the most pervasive and lethal of all brain malignancies. One factor that contributes to this poor prognosis is the highly invasive character of the tumor. GBM is characterized by microscopic infiltration of tumor cells throughout the brain, whereas non-neural metastases, as well as select lower grade gliomas, develop as self-contained and clearly delineated lesions. Illustrated by rodent xenograft tumor models as well as pathological human patient specimens, we present evidence that one fundamental switch between these two distinct pathologies--invasion and noninvasion--is mediated through the tumor extracellular matrix. Specifically, noninvasive lesions are associated with a rich matrix containing substantial amounts of glycosylated chondroitin sulfate proteoglycans (CSPGs), whereas glycosylated CSPGs are essentially absent from diffusely infiltrating tumors. CSPGs, acting as central organizers of the tumor microenvironment, dramatically influence resident reactive astrocytes, inducing their exodus from the tumor mass and the resultant encapsulation of noninvasive lesions. Additionally, CSPGs induce activation of tumor-associated microglia. We demonstrate that the astrogliotic capsule can directly inhibit tumor invasion, and its absence from GBM presents an environment favorable to diffuse infiltration. We also identify the leukocyte common antigen-related phosphatase receptor (PTPRF) as a putative intermediary between extracellular glycosylated CSPGs and noninvasive tumor cells. In all, we present CSPGs as critical regulators of brain tumor histopathology and help to clarify the role of the tumor microenvironment in brain tumor invasion. | Immunohistochemistry | | 24068827
 |
Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice.
Yi, JH; Katagiri, Y; Susarla, B; Figge, D; Symes, AJ; Geller, HM
The Journal of comparative neurology
520
3295-313
2012
Show Abstract
Chondroitin sulfate proteoglycans (CSPGs) play a pivotal role in many neuronal growth mechanisms including axon guidance and the modulation of repair processes following injury to the spinal cord or brain. Many actions of CSPGs in the central nervous system (CNS) are governed by the specific sulfation pattern on the glycosaminoglycan (GAG) chains attached to CSPG core proteins. To elucidate the role of CSPGs and sulfated GAG chains following traumatic brain injury (TBI), controlled cortical impact injury of mild to moderate severity was performed over the left sensory motor cortex in mice. Using immunoblotting and immunostaining, we found that TBI resulted in an increase in the CSPGs neurocan and NG2 expression in a tight band surrounding the injury core, which overlapped with the presence of 4-sulfated CS GAGs but not with 6-sulfated GAGs. This increase was observed as early as 7 days post injury (dpi), and persisted for up to 28 dpi. Labeling with markers against microglia/macrophages, NG2+ cells, fibroblasts, and astrocytes showed that these cells were all localized in the area, suggesting multiple origins of chondroitin-4-sulfate increase. TBI also caused a decrease in the expression of aggrecan and phosphacan in the pericontusional cortex with a concomitant reduction in the number of perineuronal nets. In summary, we describe a dual response in CSPGs whereby they may be actively involved in complex repair processes following TBI. | Immunofluorescence | Mouse | 22628090
 |
Chondroitinase and growth factors enhance activation and oligodendrocyte differentiation of endogenous neural precursor cells after spinal cord injury.
Karimi-Abdolrezaee, S; Schut, D; Wang, J; Fehlings, MG
PloS one
7
e37589
2012
Show Abstract
The adult spinal cord harbours a population of multipotent neural precursor cells (NPCs) with the ability to replace oligodendrocytes. However, despite this capacity, proliferation and endogenous remyelination is severely limited after spinal cord injury (SCI). In the post-traumatic microenvironment following SCI, endogenous spinal NPCs mainly differentiate into astrocytes which could contribute to astrogliosis that exacerbate the outcomes of SCI. These findings emphasize a key role for the post-SCI niche in modulating the behaviour of spinal NPCs after SCI. We recently reported that chondroitin sulphate proteoglycans (CSPGs) in the glial scar restrict the outcomes of NPC transplantation in SCI by reducing the survival, migration and integration of engrafted NPCs within the injured spinal cord. These inhibitory effects were attenuated by administration of chondroitinase (ChABC) prior to NPC transplantation. Here, in a rat model of compressive SCI, we show that perturbing CSPGs by ChABC in combination with sustained infusion of growth factors (EGF, bFGF and PDGF-AA) optimize the activation and oligodendroglial differentiation of spinal NPCs after injury. Four days following SCI, we intrathecally delivered ChABC and/or GFs for seven days. We performed BrdU incorporation to label proliferating cells during the treatment period after SCI. This strategy increased the proliferation of spinal NPCs, reduced the generation of new astrocytes and promoted their differentiation along an oligodendroglial lineage, a prerequisite for remyelination. Furthermore, ChABC and GF treatments enhanced the response of non-neural cells by increasing the generation of new vascular endothelial cells and decreasing the number of proliferating macrophages/microglia after SCI. In conclusions, our data strongly suggest that optimization of the behaviour of endogenous spinal NPCs after SCI is critical not only to promote endogenous oligodendrocyte replacement, but also to reverse the otherwise detrimental effects of their activation into astrocytes which could negatively influence the repair process after SCI. | | Rat | 22629425
 |
Elimination of breast tumor-associated chondroitin sulfate promotes metastasis.
R D Prinz,C M Willis,A Viloria-Petit,M Klüppel
Genetics and molecular research : GMR
10
2011
Show Abstract
Breast cancer is one of the leading causes of cancer-related deaths amongst women in the USA. The tumor microenvironment has been suggested to be an attractive therapeutic target for treatment of cancers. The glycosaminoglycan chondroitin sulfate, as part of the cellular microenvironment, consists of long linear chains of repeating disaccharide units, which are covalently attached to core proteins to form chondroitin sulfate-proteoglycans. In vitro studies have implicated chondroitin sulfate in various aspects of carcinogenesis, whereas the in vivo roles of chondroitin sulfate are less clear. Drastically elevated levels of chondroitin sulfate have been observed within the stromal compartment of many solid tumors, including human breast carcinomas, the significance of which is unknown. We examined the role of tumor-associated chondroitin sulfate in breast cancer progression. Enzymatic elimination of endogenous chondroitin sulfate by intra-tumor injections of chondroitinase ABC leads to the development of secondary tumors and increased lung metastases, while primary orthotopic tumor growth was not affected. These results establish a metastasis-inhibiting effect of primary breast tumor-associated chondroitin sulfate, which may open novel carbohydrate-based therapeutic strategies to combat breast cancer. | | | 22183949
 |
Axonal Regrowth after Spinal Cord Injury via Chondroitinase and the Tissue Plasminogen Activator (tPA)Plasmin System.
Bukhari N, Torres L, Robinson JK, Tsirka SE.
The Journal of neuroscience : the official journal of the Society for Neuroscience
31
14931-43
2011
Show Abstract
Spinal cord injury (SCI) causes permanent debilitation due to the inability of axons to grow through established scars. Both the sugar chains and core proteins of chondroitin sulfate proteoglycans (CSPGs) are inhibitory for neurite regrowth. Chondroitinase ABC (ChABC) degrades the sugar chains and allows for synaptic plasticity, suggesting that after the sugar chain cleavage additional steps occur promoting a permissive microenvironment in the glial scar region. We report that the clearance of the core protein by the tissue plasminogen activator (tPA)/plasmin proteolytic system partially contributes to ChABC-promoted plasticity. tPA and plasmin are upregulated after SCI and degrade the deglycosylated CSPG proteins. Mice lacking tPA (tPA(-/-)) exhibit attenuated neurite outgrowth and blunted sensory and motor recovery despite ChABC treatment. Coadministration of ChABC and plasmin enhanced the tPA(-/-) phenotype and supported recovery in WT SCI mice. Collectively, these findings show that the tPA/plasmin cascade may act downstream of ChABC to allow for synergistic sensory and motor improvement compared with each treatment alone and suggest a potential new approach to enhance functional recovery after SCI. | | | 22016526
 |
Efficient secretion of biologically active Chondroitinase ABC from mammalian cells in the absence of an N-terminal signal peptide.
Michael Klüppel
Molecular and cellular biochemistry
351
2011
Show Abstract
Proteoglycans carrying chondroitin sulfate side chains have been shown to fulfill important biological functions in development, disease, and signaling. One area of considerable interest is the functional importance of chondroitin sulfates as inhibitors of the regeneration of axonal projections in the mammalian central nervous system. In animal models of spinal cord injury, injections of the enzyme Chondroitinase ABC from the bacterium Proteus vulgaris into the lesion site leads to degradation of chondroitin sulfates, and promotes axonal regeneration and significant functional recovery. Here, a mammalian expression system of an epitope-tagged Chondroitinase ABC protein is described. It is demonstrated that the addition of a eukaryotic secretion signal sequence to the N-terminus of the bacterial Chondroitinase ABC sequence allowed secretion, but interfered with function of the secreted enzyme. In contrast, expression of the Chondroitinase ABC gene without N-terminal eukaryotic secretion sequence or bacterial hydrophobic leader sequence led to efficient secretion of a biologically active Chondroitinase ABC protein from both immortalized and primary cells. Moreover, the C-terminal epitope tag could be utilized to follow expression of this protein. This novel Chondroitinase ABC gene is a valuable tool for a better understanding of the in vivo roles of chondroitin sulfates in mammalian development and disease, as well as in gene therapy approaches, including the treatment of spinal chord injuries. | | | 21213020
 |