647925 Sigma-AldrichTrichostatin A, Streptomyces sp. - CAS 58880-19-6 - Calbiochem
Trichostatin A, Streptomyces sp., CAS 58880-19-6, is a cell-permeable, potent, reversible inhibitor of histone deacetylase (IC50 = 6, 38, and 6 nM for HDAC1, 4, and 6, respectively).
More>> Trichostatin A, Streptomyces sp., CAS 58880-19-6, is a cell-permeable, potent, reversible inhibitor of histone deacetylase (IC50 = 6, 38, and 6 nM for HDAC1, 4, and 6, respectively). Less<<Synonyms: 4,6-Dimethyl-7-[p-dimethylaminophenyl]-7-oxahepta-2,4-dienohydroxamic Acid, TSA, HDAC Inhibitor IX
Recommended Products
Áttekintés
| Replacement Information |
|---|
Kulcsspecifikációk táblázata
| CAS # | Empirical Formula |
|---|---|
| 58880-19-6 | C₁₇H₂₂N₂O₃ |
Products
| Katalógusszám | Csomagolás | Menny./csomag | |
|---|---|---|---|
| 647925-1MG | Muanyagampulla | 1 mg |
| Description | |
|---|---|
| Overview | A potent and reversible, cell-permeable inhibitor of histone deacetylase. Blocks cell cycle progression at the G1 phase in HeLa cells and induces a 12-fold increase in intracellular levels of gelsolin. Induces reversion of oncogenic ras-transformed NIH/3T3 cells to a normal morphology. Inhibits IL-2 gene expression (IC50 = 73 nM) in Jurkat cells and shows immunosuppressive activity in a mouse model. Down-regulates p57kip2 in Hep 3B cells. IC50 = 6 nM for HDAC1; 38 nM for HDAC4, and 8.6 nM for HDAC6A. 10 mM (500 µg/165 µl) solution of Trichostatin A, Streptomyces sp. (Cat. No. 647926) in DMSO is also available. |
| Catalogue Number | 647925 |
| Brand Family | Calbiochem® |
| Synonyms | 4,6-Dimethyl-7-[p-dimethylaminophenyl]-7-oxahepta-2,4-dienohydroxamic Acid, TSA, HDAC Inhibitor IX |
| Product Information | |
|---|---|
| CAS number | 58880-19-6 |
| ATP Competitive | N |
| Form | Off-white lyophilized solid |
| Hill Formula | C₁₇H₂₂N₂O₃ |
| Chemical formula | C₁₇H₂₂N₂O₃ |
| Reversible | Y |
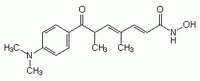
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | histone deactylase |
| Primary Target IC<sub>50</sub> | 73 nM inhibiting IL-2 gene expressionin Jurkat cells |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | MI5215000 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalógusszám | GTIN |
| 647925-1MG | 07790788052003 |
Documentation
Trichostatin A, Streptomyces sp. - CAS 58880-19-6 - Calbiochem MSDS
| Title |
|---|
Trichostatin A, Streptomyces sp. - CAS 58880-19-6 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 647925 |
References
| Hivatkozások áttekintése |
|---|
| Furumai, R., et al. 2001. Proc. Natl. Acad. Sci. USA 98, 87. Gray, S.G. and Ekstrom, T.J. 1998. Biochem. Biophys. Res. Commun. 245, 423. Takahashi, I., et al. 1996. J. Antibiot. 49, 453. Taunton, J., et al. 1996. Science 272, 408. Futamura, M., et al. 1995. Oncogene 10, 1119. Hoshikawa, Y., et al. 1994. Exp. Cell Res. 214, 189. |
Brochure
| Title |
|---|
| Pathways and Biomarkers of Toll-like Receptor (TLR) Signaling |
Technical Info
| Title |
|---|
| White Paper - The Message in the Marks: Deciphering Cancer Epigenetics |
Citations
| Titulus | |
|---|---|
|
|
| Data Sheet | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|