530510 Sigma-AldrichOxPhos Inhibitor, VLX600 - Calbiochem
A cell-permeable compound with cytotoxicity towards quiescent cells in colon cancer 3-D microtissues (~ 6 µM). Reduces mitochondrial oxidative phosphorylation in tumor cells.
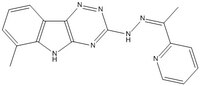
More>> A cell-permeable compound with cytotoxicity towards quiescent cells in colon cancer 3-D microtissues (~ 6 µM). Reduces mitochondrial oxidative phosphorylation in tumor cells. Less<<Synonyms: 1-(2-Pyridinyl)ethanone(6-methyl-5H-[1,2,4]triazino[5,6-b]indol-3-yl)hydrazone, (Z)-6-Methyl-3-(2-(1-(pyridin-2-yl)ethylidene)hydrazinyl)-5H-[1,2,4]triazino[5,6-b]indole, 6-Methyl-3-((2Z)-2-(1-(2-pyridinyl)ethylidene)hydrazino)-5H-[1,2,4]triazino[5,6-b]indole, VLX-600
Recommended Products
Áttekintés
| Replacement Information |
|---|
Kulcsspecifikációk táblázata
| Empirical Formula |
|---|
| C₁₇H₁₅N₇ |
Products
| Katalógusszám | Csomagolás | Menny./csomag | |
|---|---|---|---|
| 5.30510.0001 | Üvegpalack | 10 mg |
| References | |
|---|---|
| References | Zhang, X., et al. 2014. Nat. Comm. 5, 3295. |
| Product Information | |
|---|---|
| Form | Dark yellow powder |
| Hill Formula | C₁₇H₁₅N₇ |
| Chemical formula | C₁₇H₁₅N₇ |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | mitochondria |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalógusszám | GTIN |
| 5.30510.0001 | 04055977260847 |
Documentation
OxPhos Inhibitor, VLX600 - Calbiochem MSDS
| Title |
|---|
References
| Hivatkozások áttekintése |
|---|
| Zhang, X., et al. 2014. Nat. Comm. 5, 3295. |