475843 Sigma-AldrichMinocycline, Hydrochloride - CAS 13614-98-7 - Calbiochem
Semi-synthetic tetracycline derivative effective against tetracycline-resistant staphylococci.
More>> Semi-synthetic tetracycline derivative effective against tetracycline-resistant staphylococci. Less<<Synonyms: 7-Dimethylamino-6-demethyl-6-deoxytetracycline, HCl
Recommended Products
Áttekintés
| Replacement Information |
|---|
Kulcsspecifikációk táblázata
| CAS # | Empirical Formula |
|---|---|
| 13614-98-7 | C₂₃H₂₇N₃O₇ · HCl |
| Product Information | |
|---|---|
| CAS number | 13614-98-7 |
| ATP Competitive | N |
| Form | Dark yellow solid |
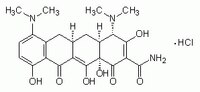
| Hill Formula | C₂₃H₂₇N₃O₇ · HCl |
| Chemical formula | C₂₃H₂₇N₃O₇ · HCl |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Metalloproteinase (MMP) |
| Primary Target IC<sub>50</sub> | 290 µM against MMP-3 |
| Primary Target K<sub>i</sub> | 13.4 nM against PARP-1 |
| Purity | ≥98% by TLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | QI7630500 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalógusszám | GTIN |
| 475843 | 0 |
Documentation
Minocycline, Hydrochloride - CAS 13614-98-7 - Calbiochem MSDS
| Title |
|---|
Minocycline, Hydrochloride - CAS 13614-98-7 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 475843 |
References
| Hivatkozások áttekintése |
|---|
| Conrad, C.A., et al. 2006. Proc. Natl. Acad, Sci. USA 103, 9685. Gilbertson-Breading, S., et al. 1995. Cancer Chemother. Pharmacol. 36, 418. Kloppenburg, M., et al. 1995. Clin. Exp. Immunol. 102, 635. Preus, H.R., et al. 1995. J. Clin. Periodontol. 22, 380. Weingart, J.D., et al. 1995. J. Neurosurg. 82, 635. Tamargo, R.J., et al. 1991. Cancer Res. 51, 672. |