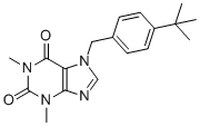
530923 Sigma-AldrichKir6.2/SUR1 Channel Activator, VU0071063 - CAS 333415-38-6 - Calbiochem
A fast acting, reversible, and selective activator of Kir6.2/SUR1 channels (EC₅₀ = 7 µM).
More>> A fast acting, reversible, and selective activator of Kir6.2/SUR1 channels (EC₅₀ = 7 µM). Less<<Synonyms: 7-(4-(tert-Butyl)benzyl)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione, Kir6.2/SUR1 Channel Opener, SUR1/Kir6.2 selective K-ATP Channel Opener, 1,3-Dimethyl-7-(4-(2-methyl-2-propanyl)benzyl)-3,7-dihydro-1H-purine-2,6-dione
Recommended Products
Áttekintés
| Replacement Information |
|---|
Kulcsspecifikációk táblázata
| CAS # | Empirical Formula |
|---|---|
| 333415-38-6 | C₁₈H₂₂N₄O₂ |
Products
| Katalógusszám | Csomagolás | Menny./csomag | |
|---|---|---|---|
| 5.30923.0001 | Üvegpalack | 10 mg |
| References | |
|---|---|
| References | Raphemot, R., et al. 2014. Mol. Pharmacol. 85, 858. |
| Product Information | |
|---|---|
| CAS number | 333415-38-6 |
| Form | White powder |
| Hill Formula | C₁₈H₂₂N₄O₂ |
| Chemical formula | C₁₈H₂₂N₄O₂ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | SUR1-containing channels |
| Primary Target IC<sub>50</sub> | EC₅₀ = 7 µM for Kir6.2/SUR1 channel activation |
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalógusszám | GTIN |
| 5.30923.0001 | 04055977243024 |
Documentation
Kir6.2/SUR1 Channel Activator, VU0071063 - CAS 333415-38-6 - Calbiochem MSDS
| Title |
|---|
References
| Hivatkozások áttekintése |
|---|
| Raphemot, R., et al. 2014. Mol. Pharmacol. 85, 858. |