361566 Sigma-AldrichGSK-3 Inhibitor IV, SB-216763 - CAS 280744-09-4 - Calbiochem
Recommended Products
Áttekintés
| Replacement Information |
|---|
Kulcsspecifikációk táblázata
| CAS # | Empirical Formula |
|---|---|
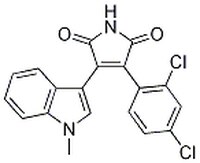
| 280744-09-4 | C₁₉H₁₂Cl₂N₂O₂ |
Products
| Katalógusszám | Csomagolás | Menny./csomag | |
|---|---|---|---|
| 361566-10MG | Üvegpalack | 10 mg |
| Product Information | |
|---|---|
| CAS number | 280744-09-4 |
| Form | Orange solid |
| Hill Formula | C₁₉H₁₂Cl₂N₂O₂ |
| Chemical formula | C₁₉H₁₂Cl₂N₂O₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalógusszám | GTIN |
| 361566-10MG | 04055977214246 |
Documentation
GSK-3 Inhibitor IV, SB-216763 - CAS 280744-09-4 - Calbiochem MSDS
| Title |
|---|
GSK-3 Inhibitor IV, SB-216763 - CAS 280744-09-4 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 361566 |
References
| Hivatkozások áttekintése |
|---|
| Gross, E., et al. 2008. Am. J. Phy. Heart Circ Physiol. 294, H1497. Bain, J., et al. 2007. Biochem. J. 408, 297. Lu, D., et al. 2004. PNAS. 101, 3118. Carmichael, J., et al. 2002. J. Biol. Chem. 277, 33791. Culbert, A.A., et al. 2001. FEBS Lett. 507, 288. Lochhead, P.A., et al. 2001. Diabetes 50, 937. Cross, D.A., et al. 2001. J. Neurochem. 77, 94. Coghlan, M.P., et al. 2000. Chem. Biol. 7, 793. |