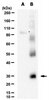
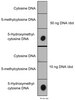
505146 Sigma-AldrichFMS/KIT Dual Kinase Inhibitor, PLX647 - Calbiochem
Synonyms: CDC2L6/CDK11/CDK19 Inhibitor, CDK8 Inhibitor, cFMS Inhibitor V, CSF-1 Receptor Inhibitor V, CSF1R Inhibitor V, DDR2 Inhibitor II, Flt-3 Inhibitor VIII, IL-34 Receptor Inhibitor V, NTRK3/TrkC Inhibitor, VEGFR Tyrosine Kinase Inhibitor XXXVI, VEGFR2 Tyrosine Kinase Inhibitor XXXIV, c-Kit Inhibitor IV, PLX-647
Recommended Products
Áttekintés
| Replacement Information |
|---|
Kulcsspecifikációk táblázata
| Empirical Formula |
|---|
| C₂₁H₁₇F₃N₄ |
| References | |
|---|---|
| References | Zhang, C., et al. 2013. Proc. Natl. Acad. Sci. USA 110, 5689. |
| Product Information | |
|---|---|
| Form | Off-white powder |
| Hill Formula | C₂₁H₁₇F₃N₄ |
| Chemical formula | C₂₁H₁₇F₃N₄ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | FMS |
| Secondary target | KIT |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalógusszám | GTIN |
| 505146 | 0 |
Documentation
FMS/KIT Dual Kinase Inhibitor, PLX647 - Calbiochem MSDS
| Title |
|---|
References
| Hivatkozások áttekintése |
|---|
| Zhang, C., et al. 2013. Proc. Natl. Acad. Sci. USA 110, 5689. |
Brochure
| Title |
|---|
| New Products Flyer- Feb 2014 - Vol 1 |