Automatic Integrity Test Instrument Qualification
Millipore validation engineers, familiar with the operation, can provide on-site assistance with performing Installation Qualification (IQ) and Operational Qualification (OQ).
Több
Millipore validation engineers, familiar with the operation, can provide on-site assistance with performing Installation Qualification (IQ) and Operational Qualification (OQ). Kevesebb
More>>
Less<<

Recommended Products
-

MAB855-2-KC Sigma-Aldrich Mouse Anti-Parainfluenza III (KC) clone 260/10B (1mL) KC -

203704 Sigma-Aldrich BPIQ-II -

MABE1064-25UG Sigma-Aldrich Anti-HsSAS-6 Antibody, clone 91.390.21 -

14-610M Sigma-Aldrich Flt-3 (D835Y) Protein, active, 250 µg -

NE1008 Sigma-Aldrich Anti-PEN-2, N-Terminal (1-26) Rabbit pAb -
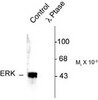
442685 Sigma-Aldrich PhosphoDetect™ Anti-MAP Kinase ERK1/ERK2 (pThr²⁰²/Tyr²⁰⁴) Rabbit pAb -
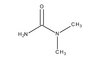
820509 Sigma-Aldrich N,N-Dimetilkarbamid -

CR0372006 Millipore Polygard-CR Cartridge Filter 20 in. 3.0 µm Code 7 Silicone -

843845 Sigma-Aldrich Szeléndiszulfid -

HTS013RTA Sigma-Aldrich Ready-to-Assay™ CCR8 Chemokine Family Receptor Frozen Cells
Overview
Specifications
Ordering Information
Documentation
Kapcsolódó termékek és alkalmazások
Product Families

Integritest® 4 Series Automated Filter Integrity Test InstrumentsEasy-to-use, portable, fully automated integrity test systemTovábbi információk >> |
Kapcsolódó termékek gyártója: Application Facete
| Disinfection control |
| Dialysis and Filtration |
Kategóriák
| Biopharmaceutical Manufacturing > Downstream Processing > Sterile Filtration > Integrity Testing |
Service Description
All of our Provantage® services include a team of experienced engineers who are personally committed to ensuring your processing success and will partner with you to overcome any challenge. This service includes qualification of filters used in your production process under worst-case conditions. It also includes application support, administrator training and assistance for performance qualification (PQ). Our Absolute Qualification Service is intended for those who are working in heavily regulated environments following cGMP guidelines.
Qualification Protocol
The protocols provided by us are designed to specific GMP guidelines and when executed by a qualified person, provide the user with results that satisfy the requirements in the guidelines.
Installation and Operational Qualification
The qualification execution includes labor (one engineer for 2 days) to execute the IQOQ protocol in the environment in which the instrument will be used. Our engineers are skilled in the operation of the equipment and provide specific instruments calibrated against national reference. After completion of the validation exercise, the data is collected and a ready to approve documentation package is provided.
Custom Service Agreements
We offer a range of qualification and preventive maintenance services to meet your unique manufacturing requirements resulting in peace of mind and maximum operational flexibility. The Integritest® 4 service agreements include inspection, calibration, maintenance and repair.
Corrective Maintenance Support
For quick resolution of any performance issues that may arise, our certified field service engineers are available to ensure that your Integritest® 4 system is up and running at optimum performance with minimum delay.
Preventive Maintenance Support
Using preventive maintenance protocols, your field service engineer will replace worn components and verify your Integritest® 4 system to ensure trouble-free operation.
How to Request Integritest® 4 System qualification protocol and services
To request on-site qualification or to get information on other validation services, call your Application Specialist or the office nearest you.






