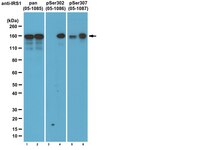
Insulin and metabolic stress stimulate multisite serine/threonine phosphorylation of insulin receptor substrate 1 and inhibit tyrosine phosphorylation.
Hançer, NJ; Qiu, W; Cherella, C; Li, Y; Copps, KD; White, MF
The Journal of biological chemistry
289
12467-84
2014
Kivonat megmutatása
IRS1 and IRS2 are key substrates of the insulin receptor tyrosine kinase. Mass spectrometry reveals more than 50 phosphorylated IRS1 serine and threonine residues (Ser(P)/Thr(P) residues) in IRS1 from insulin-stimulated cells or human tissues. We investigated a subset of IRS1 Ser(P)/Thr(P) residues using a newly developed panel of 25 phospho-specific monoclonal antibodies (αpS/TmAb(Irs1)). CHO cells overexpressing the human insulin receptor and rat IRS1 were stimulated with insulin in the absence or presence of inhibitors of the PI3K → Akt → mechanistic target of rapamycin (mTOR) → S6 kinase or MEK pathways. Nearly all IRS1 Ser(P)/Thr(P) residues were stimulated by insulin and significantly suppressed by PI3K inhibition; fewer were suppressed by Akt or mTOR inhibition, and none were suppressed by MEK inhibition. Insulin-stimulated Irs1 tyrosine phosphorylation (Tyr(P)(Irs1)) was enhanced by inhibition of the PI3K → Akt → mTOR pathway and correlated with decreased Ser(P)-302(Irs1), Ser(P)-307(Irs1), Ser(P)-318(Irs1), Ser(P)-325(Irs1), and Ser(P)-346(Irs1). Metabolic stress modeled by anisomycin, thapsigargin, or tunicamycin increased many of the same Ser(P)/Thr(P) residues as insulin, some of which (Ser(P)-302(Irs1), Ser(P)-307(Irs1), and four others) correlated significantly with impaired insulin-stimulated Tyr(P)(Irs1). Thus, IRS1 Ser(P)/Thr(P) is an integrated response to insulin stimulation and metabolic stress, which associates with reduced Tyr(P)(Irs1) in CHO(IR)/IRS1 cells. | 24652289
 |