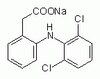
MABN878 Sigma-AldrichAnti-phospho-Amyloid beta (Ser8) Antibody, clone 1E4E11
Anti-phospho-Amyloid beta (Ser8) Antibody, clone 1E4E11 is an antibody against phospho-Amyloid beta (Ser8) for use in Western Blotting, Enzyme Immunoassay (ELISA), Immunohistochemistry, Immunofluorescence.
More>> Anti-phospho-Amyloid beta (Ser8) Antibody, clone 1E4E11 is an antibody against phospho-Amyloid beta (Ser8) for use in Western Blotting, Enzyme Immunoassay (ELISA), Immunohistochemistry, Immunofluorescence. Less<<Recommended Products
Áttekintés
| Replacement Information |
|---|
Kulcsspecifikációk táblázata
| Species Reactivity | Key Applications | Host | Format | Antibody Type |
|---|---|---|---|---|
| H | WB, ELISA, IHC, IF | M | Purified | Monoclonal Antibody |
| References |
|---|
| Product Information | |
|---|---|
| Format | Purified |
| Presentation | Purified mouse monoclonal IgG1κ antibody in buffer containing 0.1 M Tris-Glycine (pH 7.4), 150 mM NaCl with 0.05% sodium azide. |
| Quality Level | MQ100 |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Storage and Shipping Information | |
|---|---|
| Storage Conditions | Stable for 1 year at 2-8°C from date of receipt. |
| Packaging Information | |
|---|---|
| Material Size | 100 μg |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalógusszám | GTIN |
| MABN878 | 04055977350937 |
Documentation
Anti-phospho-Amyloid beta (Ser8) Antibody, clone 1E4E11 MSDS
| Title |
|---|






 Ab[MABN878_WB pAbeta (Ser8) Ab-ALL].jpg)
 Ab[MABN878_WB pAbeta (Ser8) Ab-ALL].jpg)
 Ab[MABN878_WB2 pAbeta (Ser8) Ab-ALL].jpg)