A chromatin activity-based chemoproteomic approach reveals a transcriptional repressome for gene-specific silencing.
Liu, C; Yu, Y; Liu, F; Wei, X; Wrobel, JA; Gunawardena, HP; Zhou, L; Jin, J; Chen, X
Nature communications
5
5733
2014
Kivonat megmutatása
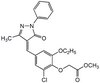
Immune cells develop endotoxin tolerance (ET) after prolonged stimulation. ET increases the level of a repression mark H3K9me2 in the transcriptionally silent chromatin specifically associated with pro-inflammatory genes. However, it is not clear what proteins are functionally involved in this process. Here we show that a novel chromatin activity-based chemoproteomic (ChaC) approach can dissect the functional chromatin protein complexes that regulate ET-associated inflammation. Using UNC0638 that binds the enzymatically active H3K9-specific methyltransferase G9a/GLP, ChaC reveals that G9a is constitutively active at a G9a-dependent mega-dalton repressome in primary endotoxin-tolerant macrophages. G9a/GLP broadly impacts the ET-specific reprogramming of the histone code landscape, chromatin remodelling and the activities of select transcription factors. We discover that the G9a-dependent epigenetic environment promotes the transcriptional repression activity of c-Myc for gene-specific co-regulation of chronic inflammation. ChaC may also be applicable to dissect other functional protein complexes in the context of phenotypic chromatin architectures. | 25502336
 |