AIM2 Drives Joint Inflammation in a Self-DNA Triggered Model of Chronic Polyarthritis.
Jakobs, C; Perner, S; Hornung, V
PloS one
10
e0131702
2015
Kivonat megmutatása
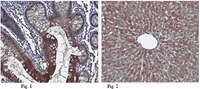
Mice lacking DNase II display a polyarthritis-like disease phenotype that is driven by translocation of self-DNA into the cytoplasm of phagocytic cells, where it is sensed by pattern recognition receptors. While pro-inflammatory gene expression is non-redundantly linked to the presence of STING in these mice, the contribution of the inflammasome pathway has not been explored. To this end, we studied the role of the DNA-sensing inflammasome receptor AIM2 in this self-DNA driven disease model. Arthritis-prone mice lacking AIM2 displayed strongly decreased signs of joint inflammation and associated histopathological findings. This was paralleled with a reduction of caspase-1 activation and pro-inflammatory cytokine production in diseased joints. Interestingly, systemic signs of inflammation that are associated with the lack of DNase II were not dependent on AIM2. Taken together, these data suggest a tissue-specific role for the AIM2 inflammasome as a sensor for endogenous DNA species in the course of a ligand-dependent autoinflammatory condition. | 26114879
 |
Viperin is highly induced in neutrophils and macrophages during acute and chronic lymphocytic choriomeningitis virus infection.
Hinson, Ella R, et al.
J. Immunol., 184: 5723-31 (2010)
2009
Kivonat megmutatása
Although most cells are thought to respond to IFNs, there is limited information regarding specific cells that respond in vivo. Viperin is an IFN-induced antiviral protein and, therefore, is an excellent marker for IFN-responsive cells. In this study, we analyzed viperin expression in vivo during acute lymphocytic choriomeningitis virus Armstrong infection, which induces high levels of type I IFNs, and in persistently infected lymphocytic choriomeningitis virus carrier mice, which contain low levels of type I IFNs. Viperin was induced in lymphoid cells and dendritic cells (DCs) during acute infection and highly induced in neutrophils and macrophages. The expression kinetics in neutrophils, macrophages, and T and B cells paralleled IFN-alpha levels, but DCs expressed viperin with delayed kinetics. In carrier mice, viperin was expressed in neutrophils and macrophages but not in T and B cells or DCs. For acutely infected and carrier mice, viperin expression was IFN dependent, because treating type I IFNR knockout mice with IFN-gamma-neutralizing Abs inhibited viperin expression. Viperin localized to the endoplasmic reticulum and lipid droplet-like vesicles in neutrophils. These findings delineate the kinetics and cells responding to IFNs in vivo and suggest that the profile of IFN-responsive cells changes in chronic infections. Furthermore, these data suggest that viperin may contribute to the antimicrobial activity of neutrophils. | 20410488
 |
The N-terminal amphipathic alpha-helix of viperin mediates localization to the cytosolic face of the endoplasmic reticulum and inhibits protein secretion.
Hinson, Ella R and Cresswell, Peter
J. Biol. Chem., 284: 4705-12 (2009)
2009
Kivonat megmutatása
Viperin is an evolutionarily conserved interferon-inducible protein that localizes to the endoplasmic reticulum (ER) and inhibits a number of DNA and RNA viruses. In this study, we report that viperin specifically localizes to the cytoplasmic face of the ER and that an amphipathic alpha-helix at its N terminus is necessary for the ER localization of viperin and sufficient to promote ER localization of a reporter protein, dsRed. Overexpression of intact viperin but not the amphipathic alpha-helix fused to dsRed induced crystalloid ER. Consistent with other proteins that induce crystalloid ER, viperin self-associates, and it does so independently of the amphipathic alpha-helix. Viperin expression also affected the transport of soluble but not membrane-associated proteins. Expression of intact viperin or an N-terminal alpha-helix-dsRed fusion protein significantly reduced secretion of soluble alkaline phosphatase and reduced its rate of ER-to-Golgi trafficking. Similarly, viperin expression inhibited bulk protein secretion and secretion of endogenous alpha(1)-antitrypsin and serum albumin from HepG2 cells. Converting hydrophobic residues in the N-terminal alpha-helix to acidic residues partially or completely restored normal transport of soluble alkaline phosphatase, suggesting that the extended amphipathic nature of the N-terminal alpha-helical domain is essential for inhibiting protein secretion. | 19074433
 |