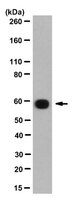
Stage-specific expression of the immunophilin FKBP59 messenger ribonucleic acid and protein during differentiation of male germ cells in rabbits and rats.
Sananes, N; Baulieu, EE; Le Goascogne, C
Biology of reproduction
58
353-60
1998
Kivonat megmutatása
FKBP59 is an immunophilin that binds the immunosuppressant drugs FK506 and rapamycin. It is a 90-kDa heat shock protein (hsp90)-binding protein that was originally discovered as a member of steroid receptor complexes. FKBP59 is ubiquitous and well conserved, and it appears to be a multifunctional protein. It has peptidylprolyl cis-trans-isomerase activity and therefore may be involved in protein folding as a molecular chaperon. FKBP59 also includes in its structure a tetratricopeptide repeat (TPR) motif and could have a function in the cell division process. In situ hybridization experiments revealed an overexpression of FKBP59 mRNA in rabbit and rat testes in comparison with other organs. This high level of expression was restricted to germ cells of the seminiferous epithelium. Increasing levels of FKBP59 mRNA became obvious from the midpachytene stage, and the strongest signal was observed in the late pachytene, diplotene, and diakinesis primary spermatocytes. The expression then declined progressively in postmeiotic early spermatids. High expression of FKBP59 mRNA did not occur in earlier and later germ cell stages. During prepubertal development, Northern blot and in situ hybridization of rat testes examined at various postnatal ages revealed that FKBP59 mRNA was not expressed at over a basal level until the pachytene stage. High expression of the FKBP59 protein was demonstrated in the rabbit testis by Western blot and was localized by immunohistochemistry from late pachytene spermatocytes to round spermatids. The cell type-specific and developmental stage-specific expression of FKBP59 at a restricted period of male germ cell differentiation suggests that FKBP59 is involved in a specific function during the cell division process. | 9475389
 |
Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex.
Tai, PK; Albers, MW; Chang, H; Faber, LE; Schreiber, SL
Science (New York, N.Y.)
256
1315-8
1992
Kivonat megmutatása
Immunophilins, a family of proteins that exhibit rotamase (peptidyl-prolyl cis-trans isomerase) activity in vitro, are expressed in many organisms and most tissues. Although some immunophilins can mediate the immunosuppressive actions of FK506, rapamycin, and cyclosporin A, the physiological role of the unligated proteins is not known. A 59-kilodalton member of the FK506- and rapamycin-binding class was found to associate in the absence of these drugs with two heat shock proteins (hsp90 and hsp70) and the glucocorticoid receptor (GR). Together, these proteins make up the inactive GR, thus biochemically linking two families of proteins proposed to be involved in protein folding and assembly as well as two potent immunosuppressive modalities. | 1376003
 |
Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes.
Sanchez, ER
The Journal of biological chemistry
265
22067-70
1990
Kivonat megmutatása
The recently-described p59 protein has been shown to be associated with untransformed steroid receptors present in rabbit uterus and rat liver cytosols (Tai, P. K., Maeda, Y., Nakao, K., Wakim, N. G., Duhring, J. L., and Faber, L. E. (1986) Biochemistry 25, 5269-5275; Renoir, J.-M., Radanyi, C., Faber, L. E., and Baulieu, E.-E. (1990) J. Biol. Chem. 265, 10740-10745), while a smaller version of this protein (p56) interacts with glucocorticoid receptors in human IM-9 cell cytosols (Sanchez, E. R., Faber, L. E., Henzel, W. J., and Pratt, W. B. (1990) Biochemistry 29, 5145-5152). In addition to interacting with glucocorticoid receptors, the p56 protein of IM-9 cell cytosol is also found as part of a large heteromeric complex that contains both the 70-kDa and 90-kDa heat shock proteins (hsp70 and hsp90, respectively). Given this association of p56 with the two major stress proteins, I have speculated that p56 may itself be a heat shock protein. In this paper, the effect of heat stress on the rate of synthesis of p56 is determined. Intact IM-9 cells were exposed to 37 or 43 degrees C for 4 h, followed by pulse-labeling with [35S]methionine. Analysis of whole cytosolic extracts by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography reveal an increased rate of radiolabeling for hsp70, hsp90, hsp100, ad hsp110, but no heat-inducible protein of smaller relative molecular mass is detected. However, immune-purification of p56 from normal and heat-stressed cytosols with the EC1 monoclonal antibody results in the presence of a 56-kDa protein that exhibits an increased rate of synthesis in response to heat stress. The results of two-dimensional gel Western blots employing the EC1 antibody demonstrate that this heat-inducible protein is indeed the EC1-reactive p56 protein and that the induction effect is not due to unequal yields of p56 during immune-purification. Heat stress has no effect on the composition of the p56.hsp.70.hsp90 complex, except that the complex derived from heat shocked-cells contains both the constitutive and heat-inducible forms of hsp70. Induction of p56 also occurs in IM-9 cells subjected to chemical stress (sodium arsenite). It is proposed that p56 is a steroid receptor-associated heat shock protein which can now be termed hsp56. Like hsp90, hsp56 likely serves in some vital cellular role apart from any specific function it provides in steroid receptor action. | 2266108
 |
Nuclear localization of two steroid receptor-associated proteins, hsp90 and p59.
Gasc, JM; Renoir, JM; Faber, LE; Delahaye, F; Baulieu, EE
Experimental cell research
186
362-7
1990
Kivonat megmutatása
It has been proposed that the unliganded nontransformed form of steroid hormone receptor is a heterooligomer comprising, in addition to the hormone-binding subunit, two associated proteins: a heat shock protein of MW 90,000 (hsp90) and another protein of MW 59,000 (p59). Using monoclonal antibodies, we demonstrate immunocytochemically the presence of both hsp90 and p59 in cell nuclei of progesterone target cells of the rabbit uterus. While steroid receptors (e.g., progesterone receptors) appear to be exclusively nuclear, we find p59 predominantly in the cell nuclei and hsp90 in both the nucleus and the cytoplasm. In addition, Western blotting of high-salt extracts of nuclear proteins detects the presence of hsp90 and p59 in the nuclei of rabbit uterus. These observations are consistent with the presence of the untransformed heterooligomeric form of steroid hormone receptors in the nuclei of target cells. | 2298246
 |
The 56-59-kilodalton protein identified in untransformed steroid receptor complexes is a unique protein that exists in cytosol in a complex with both the 70- and 90-kilodalton heat shock proteins.
Sanchez, ER; Faber, LE; Henzel, WJ; Pratt, WB
Biochemistry
29
5145-52
1990
Kivonat megmutatása
It has previously been shown that 9S, untransformed progestin, estrogen, androgen, and glucocorticoid receptor complexes in rabbit uterine and liver cytosols contain a 59-kDa protein [Tai, P. K., Maeda, Y., Nakao, K., Wakim, N. G., Duhring, J. L., & Faber, L. E. (1986) Biochemistry 25, 5269-5275]. In this work we show that the monoclonal antibody KN 382/EC1 raised against the rabbit 59-kDa protein reacts with 9S, untransformed glucocorticoid receptor complexes in cytosol prepared from human IM-9 lymphocytes but not with 4S salt-transformed receptors. The human protein recognized by the EC1 antibody is a 56-kDa protein (p56) of moderate abundance located predominantly in the cytoplasm by indirect immunofluorescence. There are at least six isomorphs of p56 by two-dimensional gel analysis. N-Terminal sequencing (20 amino acids) shows that p56 is a unique human protein. When p56 is immunoadsorbed from IM-9 cell cytosol, both the 70- and 90-kDa heat shock proteins are coadsorbed in an immune-specific manner. Neither heat shock protein reacts directly with the EC1 antibody. We conclude that p56 exists in cytosol in a higher order complex containing hsp70 and hsp90, both of which in turn have been found to be associated with untransformed steroid receptors. | 2378870
 |