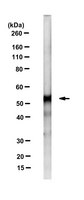
Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis.
BasuRay, S; Wang, Y; Smagris, E; Cohen, JC; Hobbs, HH
Proc Natl Acad Sci U S A
116
9521-9526
2019
Kivonat megmutatása
Fatty liver disease (FLD) is a disorder in which accumulation of triglycerides (TGs) in the liver can lead to inflammation, fibrosis, and cirrhosis. Previously, we identified a variant (I148M) in patatin-like phospholipase domain-containing protein 3 (PNPLA3) that is strongly associated with FLD, but the mechanistic basis for the association remains elusive. Although PNPLA3 has TG hydrolase activity in vitro, inactivation or overexpression of the WT protein in mice does not cause steatosis. In contrast, expression of two catalytically defective forms of PNPLA3 (I148M or S47A) in sucrose-fed mice causes accumulation of both PNPLA3 and TGs on hepatic lipid droplets (LDs). To determine if amassing PNPLA3 on LDs is a cause or consequence of steatosis, we engineered a synthetic isoform of PNPLA3 that uncouples protein accumulation from loss of enzymatic activity. Expression of a ubiquitylation-resistant form of PNPLA3 in mice caused accumulation of PNPLA3 on hepatic LDs and development of FLD. Lowering PNPLA3 levels by either shRNA knockdown or proteolysis-targeting chimera (PROTAC)-mediated degradation reduced liver TG content in mice overexpressing PNPLA3(148M). Taken together, our results show that the steatosis associated with PNPLA3(148M) is caused by accumulation of PNPLA3 on LDs. | 31019090
 |