Differential effects of HIF-1 inhibition by YC-1 on the overall outcome and blood-brain barrier damage in a rat model of ischemic stroke.
Yan, J; Zhou, B; Taheri, S; Shi, H
PloS one
6
e27798
2010
Kivonat megmutatása
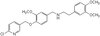
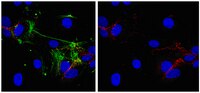
Hypoxia-inducible factor 1 (HIF-1) is a master regulator of cellular adaptation to hypoxia and has been suggested as a potent therapeutic target in cerebral ischemia. Here we show in an ischemic stroke model of rats that inhibiting HIF-1 and its downstream genes by 3-(5'-hydroxymethyl-2'-furyl)-1-benzylindazole (YC-1) significantly increases mortality and enlarges infarct volume evaluated by MRI and histological staining. Interestingly, the HIF-1 inhibition remarkably ameliorates ischemia-induced blood-brain barrier (BBB) disruption determined by Evans blue leakage although it does not affect brain edema. The result demonstrates that HIF-1 inhibition has differential effects on ischemic outcomes and BBB permeability. It indicates that HIF-1 may have different functions in different brain cells. Further analyses show that ischemia upregulates HIF-1 and its downstream genes erythropoietin (EPO), vascular endothelial growth factor (VEGF), and glucose transporter (Glut) in neurons and brain endothelial cells and that YC-1 inhibits their expression. We postulate that HIF-1-induced VEGF increases BBB permeability while certain other proteins coded by HIF-1's downstream genes such as epo and glut provide neuroprotection in an ischemic brain. The results indicate that YC-1 lacks the potential as a cerebral ischemic treatment although it confers certain protection to the cerebral vascular system. | 22110762
 |
Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination.
Walczak, P; All, AH; Rumpal, N; Gorelik, M; Kim, H; Maybhate, A; Agrawal, G; Campanelli, JT; Gilad, AA; Kerr, DA; Bulte, JW
Glia
59
499-510
2010
Kivonat megmutatása
Transplantation of glial progenitor cells results in transplant-derived myelination and improved function in rodents with genetic dysmyelination or chemical demyelination. However, glial cell transplantation in adult CNS inflammatory demyelinating models has not been well studied. Here we transplanted human glial-restricted progenitor (hGRP) cells into the spinal cord of adult rats with inflammatory demyelination, and monitored cell fate in chemically immunosuppressed animals. We found that hGRPs migrate extensively, expand within inflammatory spinal cord lesions, do not form tumors, and adopt a mature glial phenotype, albeit at a low rate. Human GRP-transplanted rats, but not controls, exhibited preserved electrophysiological conduction across the spinal cord, though no differences in behavioral improvement were noted between the two groups. Although these hGRPs myelinated extensively after implantation into neonatal shiverer mouse brain, only marginal remyelination was observed in the inflammatory spinal cord demyelination model. The low rate of transplant-derived myelination in adult rat spinal cord may reflect host age, species, transplant environment/location, and/or immune suppression regime differences. We conclude that hGRPs have the capacity to myelinate dysmyelinated neonatal rodent brain and preserve conduction in the inflammatory demyelinated adult rodent spinal cord. The latter benefit is likely dependent on trophic support and suggests further exploration of potential of glial progenitors in animal models of chronic inflammatory demyelination. | 21264955
 |
Gene array profile identifies collagen type XV as a novel human osteoblast-secreted matrix protein.
Lisignoli, Gina, et al.
J. Cell. Physiol., 220: 401-9 (2009)
2009
Kivonat megmutatása
Bone marrow stromal cells (MSCs) and osteoblasts are the two main non-haematopoietic cellular components of human bone tissue. To identify novel osteoblast-related molecules, we performed a gene expression profiling analysis comparing MSCs and osteoblasts isolated from the same donors. Genes differentially overexpressed in osteoblasts were mainly related to the negative control of cell proliferation, pro-apoptotic processes, protein metabolism and bone remodelling. Notably, we also identified the collagen XV (COL15A1) gene as the most up-regulated gene in osteoblasts compared with MSCs, previously described as being expressed in the basement membrane in other cell types. The expression of collagen type XV was confirmed at the protein level on isolated osteoblasts and we demonstrated that it significantly increases during the osteogenic differentiation of MSCs in vitro and that free ionised extracellular calcium significantly down-modulates its expression. Moreover, light and electron microscopy showed that collagen type XV is expressed in bone tissue biopsies mainly by working osteoblasts forming new bone tissue or lining bone trabeculae. To our knowledge, these data represent the first evidence of the expression of collagen type XV in human osteoblasts, a calcium-regulated protein which correlates to a specific functional state of these cells. | 19365806
 |
Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons.
Obata, F; Tohyama, K; Bonev, AD; Kolling, GL; Keepers, TR; Gross, LK; Nelson, MT; Sato, S; Obrig, TG
The Journal of infectious diseases
198
1398-406
2008
Kivonat megmutatása
Affinity-purified Shiga toxin (Stx) 2 given intraperitoneally to mice caused weight loss and hind-limb paralysis followed by death. Globotriaosylceramide (Gb(3)), the receptor for Stx2, was localized to neurons of the central nervous system (CNS) of normal mice. Gb3 was not found in astrocytes or endothelial cells of the CNS. In human cadaver CNS, we found Gb(3) in neurons and endothelial cells. Mouse Gb(3) localization was confirmed by immunoelectron microscopy. In Stx2-exposed mice, anti-Stx2-gold immunoreaction was positive in neurons. During paralysis, after Stx2 injection, multiple glial nuclei were observed surrounding motoneurons by electron microscopy. Also revealed was a lamellipodia-like process physically inhibiting the synaptic connection of motoneurons. Ca2+ imaging of cerebral astrocytic end-feet in Stx2-treated mouse brains suggested that the toxin increased neurotransmitter release from neurons. In this article, we propose that the neuron is a primary target of Stx2, affecting neuronal function and leading to paralysis. | 18754742
 |