Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice.
Kim-Muller, JY; Zhao, S; Srivastava, S; Mugabo, Y; Noh, HL; Kim, YR; Madiraju, SR; Ferrante, AW; Skolnik, EY; Prentki, M; Accili, D
Cell metabolism
20
593-602
2014
Kivonat megmutatása
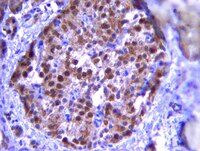
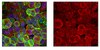
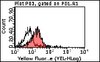
Pancreatic β cell failure in type 2 diabetes is associated with functional abnormalities of insulin secretion and deficits of β cell mass. It's unclear how one begets the other. We have shown that loss of β cell mass can be ascribed to impaired FoxO1 function in different models of diabetes. Here we show that ablation of the three FoxO genes (1, 3a, and 4) in mature β cells results in early-onset, maturity-onset diabetes of the young (MODY)-like diabetes, with abnormalities of the MODY networks Hnf4α, Hnf1α, and Pdx1. FoxO-deficient β cells are metabolically inflexible, i.e., they preferentially utilize lipids rather than carbohydrates as an energy source. This results in impaired ATP generation and reduced Ca(2+)-dependent insulin secretion. The present findings demonstrate a secretory defect caused by impaired FoxO activity that antedates dedifferentiation. We propose that defects in both pancreatic β cell function and mass arise through FoxO-dependent mechanisms during diabetes progression. | 25264246
 |
High molecular weight proteins in the nematode C. elegans bind [3H]ryanodine and form a large conductance channel.
Y K Kim,H H Valdivia,E B Maryon,P Anderson,R Coronado
Biophysical journal
63
1992
Kivonat megmutatása
Single-channel properties of a polypeptide fraction from the nematode Caenorhabditis elegans highly enriched in binding sites were studied in planar bilayers. [3H]Ryanodine binding sites were purified by sucrose gradient centrifugation of C. elegans microsomes solubilized in CHAPS detergent. The highest [3H]ryanodine binding activity sedimented at approximately 18% sucrose (wt/vol), and was composed of a major polypeptide with a M(r) of 360,000 and a minor polypeptide with a M(r) of 170,000. The ryanodine-binding polypeptide(s) formed a Ca(2+)-permeable channel with a permeability ratio P(divalent)/P(monovalent) = 4 and two conductance states of 215 pS and 78 pS in 0.25 M KCl. Ryanodine locked the channel in the 78 pS state and inhibited transitions between the 215 pS and 78 pS states. These data demonstrated the presence of a ryanodine receptor in C. elegans with functional properties comparable to those described in mammalian muscle. Teljes cikk | 1335783
 |